- Therapeutic Cataract & Refractive
- Lens Technology
- Glasses
- Ptosis
- AMD
- COVID-19
- DME
- Ocular Surface Disease
- Optic Relief
- Geographic Atrophy
- Cornea
- Conjunctivitis
- LASIK
- Myopia
- Presbyopia
- Allergy
- Nutrition
- Pediatrics
- Retina
- Cataract
- Contact Lenses
- Lid and Lash
- Dry Eye
- Glaucoma
- Refractive Surgery
- Comanagement
- Blepharitis
- OCT
- Patient Care
- Diabetic Eye Disease
- Technology
AOA releases statement regarding FDA eye drop recall
The FDA recalled 26 eye drops due to risk of infection and possible vision loss.
Use of recalled eye drops may cause sudden or persistent irritation. Consumers who own products on the FDA's recall list should dispose of the medication appropriately. (Image credit: Adobe Stock/©Alessandro Grandini)
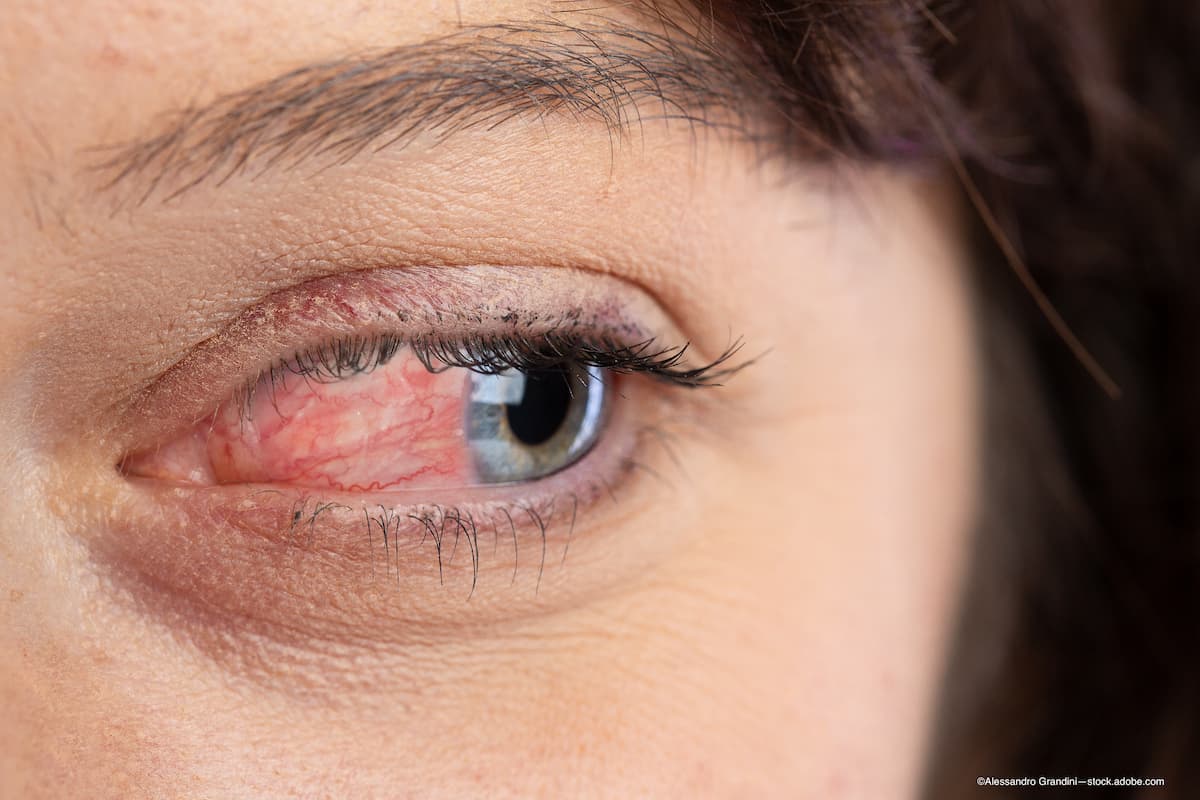
Following an alert from the US Food and Drug Administration (FDA) to halt use of 26 eye drops due to risk of infection and possible vision loss, the American Optometric Association (AOA) has released a statement as well as an infographic for circulation.
Patients who have used any products on the list or those who experience any new or prolonged eye irritation should schedule an appointment with a local optometrist. Optometrists should do their part to ask patients who present with irritation if they have used any of the listed eye drops. The AOA recommends that all consumers who have purchased the listed eye drops dispose of the products according to the FDA’s guidelines. This may require a trip to a drug take-back site.
While eye drops are safe when manufactured properly and instilled with care, there has been an uptick in contaminated eye drops over the last year. To combat this, the AOA recommends patients consult with an optometrist prior to purchase of eye drops. Optometrists should counsel patients on appropriate drop purchase and an appropriate treatment plan. During the consultation, optometrists and patients should discuss prescribed and over-the-counter medications being used to avoid contraindication and health complications.
In the interest of public health, eye care providers and patients alike are encouraged to report adverse events or quality problems to the FDA via the MedWatch Adverse Event Reporting program. This may help the FDA identify and recall contaminated eye drops, as well as other problematic medications and drugs, earlier, thus minimizing patient risk.
Optometrists who believe their patients may have purchased any of the 26 recalled eye drops may distribute this infographic created by the AOA.

