- Therapeutic Cataract & Refractive
- Lens Technology
- Glasses
- Ptosis
- AMD
- COVID-19
- DME
- Ocular Surface Disease
- Optic Relief
- Geographic Atrophy
- Cornea
- Conjunctivitis
- LASIK
- Myopia
- Presbyopia
- Allergy
- Nutrition
- Pediatrics
- Retina
- Cataract
- Contact Lenses
- Lid and Lash
- Dry Eye
- Glaucoma
- Refractive Surgery
- Comanagement
- Blepharitis
- OCT
- Patient Care
- Diabetic Eye Disease
- Technology
Imaging technology helps in scanning for retinal diseases
Advancements enable better diagnosis, treatment of patients.
Arriving at a clinical diagnosis is often like a jigsaw puzzle: pieces are the history of present illness (HPI), any contributory risk factor noted in a patient’s medical and ocular history, patient symptoms, and various signs detected during examination.
Assessing ocular posterior segment disease is similar to the assessment of many other conditions. First, we rely on our physical clinical examination. In many cases, this is sufficient to arrive at a diagnosis and create a management plan.
In other cases, we must rely on various ancillary tests. These tests usually fall into 2 categories: functional and structural. A functional example is a visual field test, whereas a structural example would be optical coherence tomography (OCT). Some of these tests also play a role in the documentation of normal vs abnormal states.

This is a review of various imaging structural techniques of the posterior segment, including fundus photography and its variations, retinal angiography, OCT, and OCT-angiography. Advances in ocular posterior segment imaging have greatly improved eye care practitioners’ (ECPs) ability not only to better understand various vitreoretinal and choroidal diseases, but also to better diagnose and manage these diseases.
Diagnostic fundus photography
Fundus photography has been around since the 1800s.1 But the evolution from film and paper to digital photography and then—leaping even further—to scanning laser ophthalmoscope (SLO) imaging has transformed fundus photography from a documentary image source to a diagnostic technology.
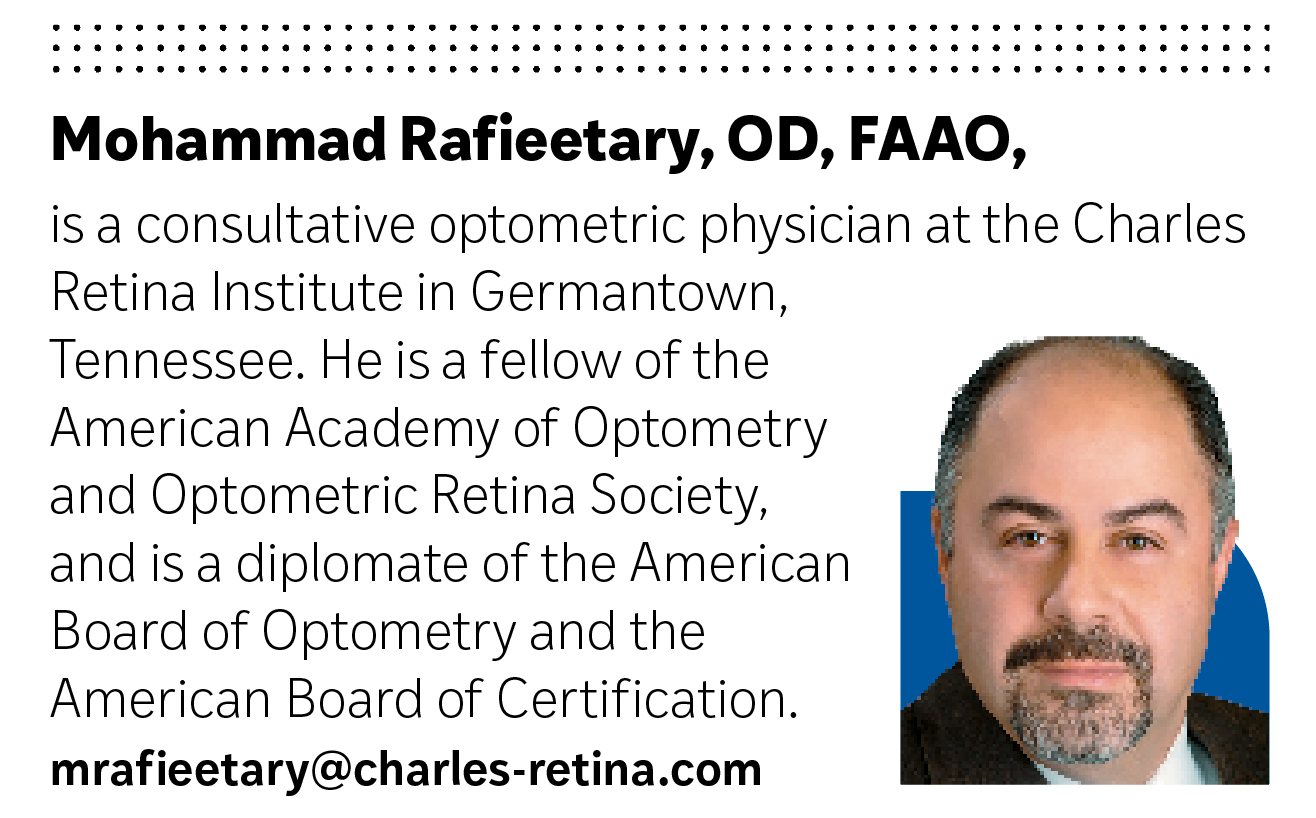
Color and multispectral SLO imaging by separating various posterior segment layers allows better detection of retinal disease than conventional white flash fundus photography2,3 (Figure 1).
In addition to improved diagnostic features over conventional white flash, SLO photography can better penetrate opacities of the optical media such as cataracts.2
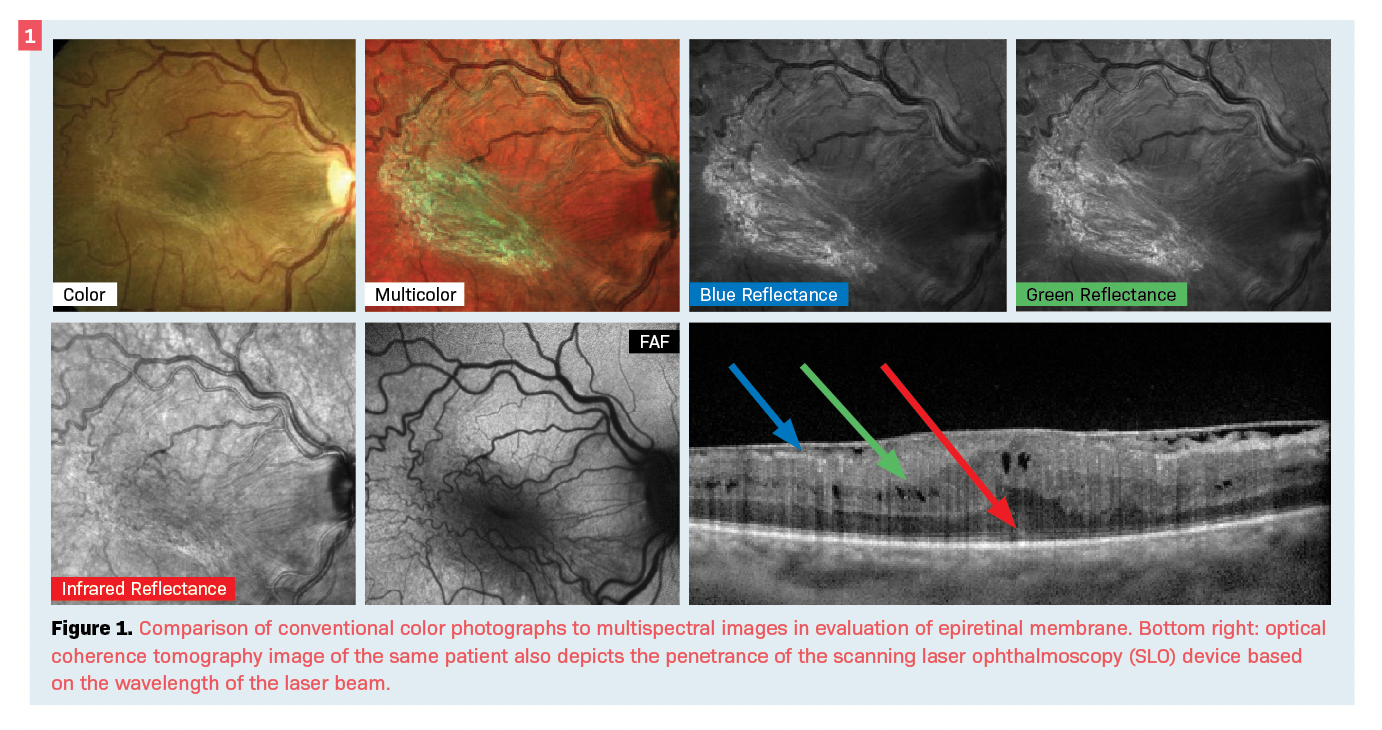
Another advancement in fundus photography is ultra-widefield (UWF) photography. By expanding the field to up to 200° in a single image, UWF fundus photography can improve the diagnosis of peripheral retinal lesions—as well as common conditions such as diabetic retinopathy—over the Early Treatment Diabetic Retinopathy Study (ETDRS) 7-field standard (see Figure 2).4-6
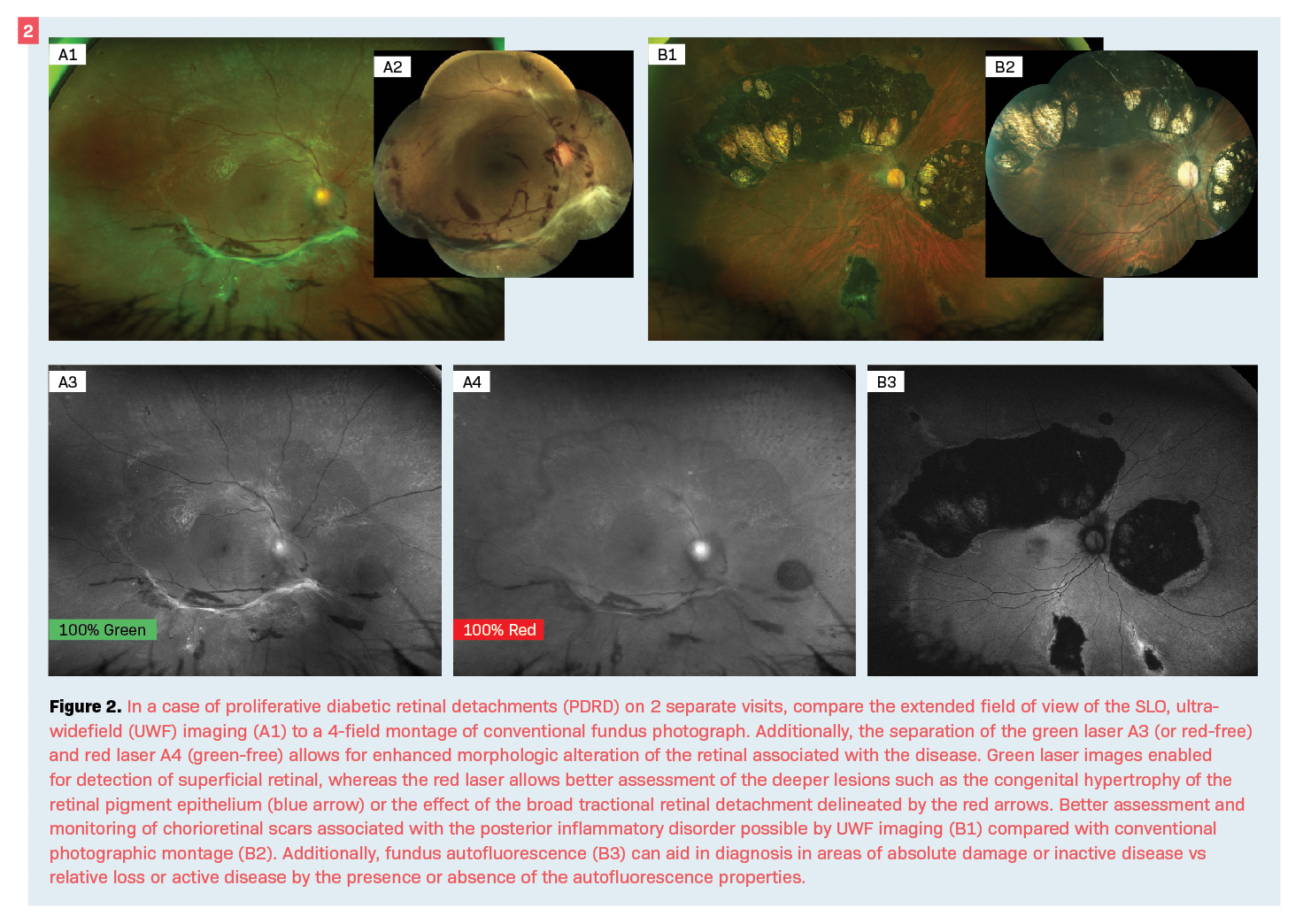
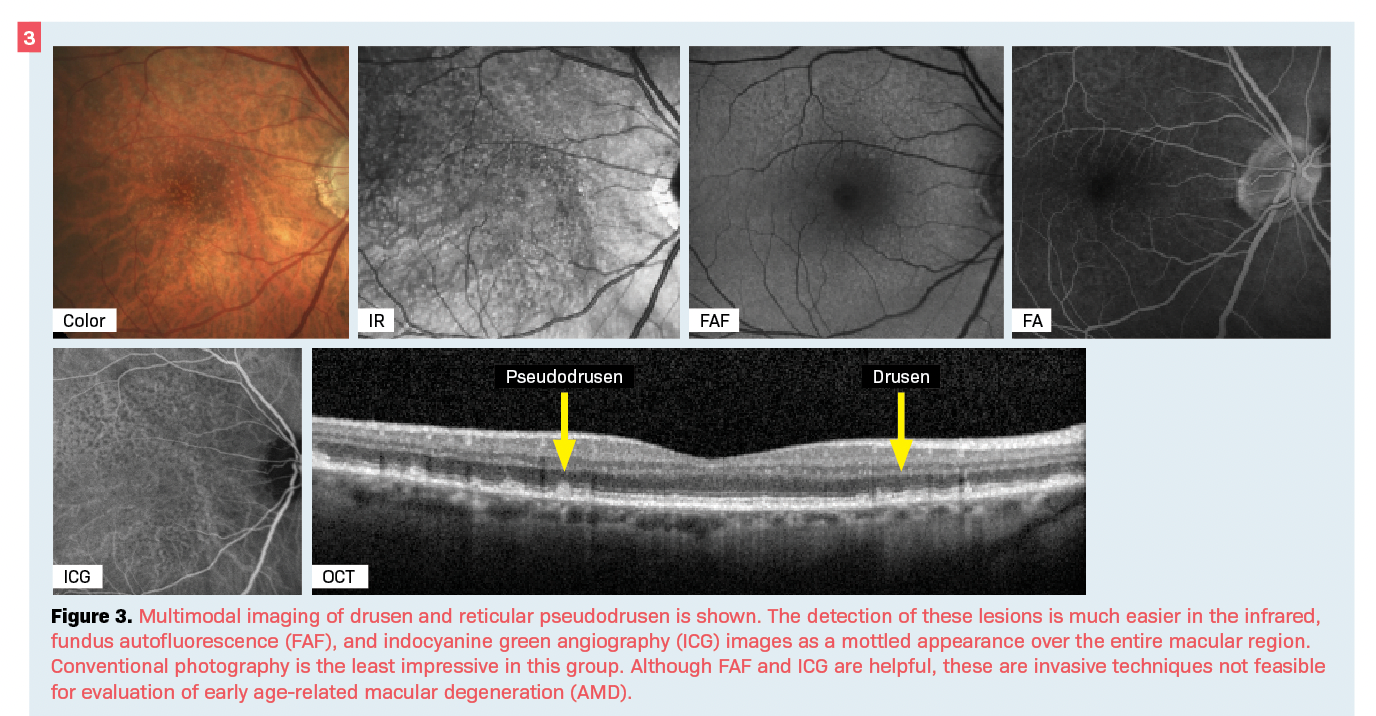
Other features of diagnostic fundus photography are infrared (IR) or near-infrared (NIR) reflectance and fundus autofluorescence (FAF). It is thought that IR photos may be more sensitive in detecting lesions such as drusen and reticular pseudodrusen findings commonly associated with age-related macular degeneration (AMD) that may be missed in clinical examination (Figure 3).7,8
FAF is a noninvasive photography technique that is gaining traction, particularly for diagnosing macular diseases such as inherited macular dystrophies, geographic atrophy (GA) associated with AMD, hydroxychloroquine maculopathy, and central serous chorioretinopathy (Figure 4).9-13
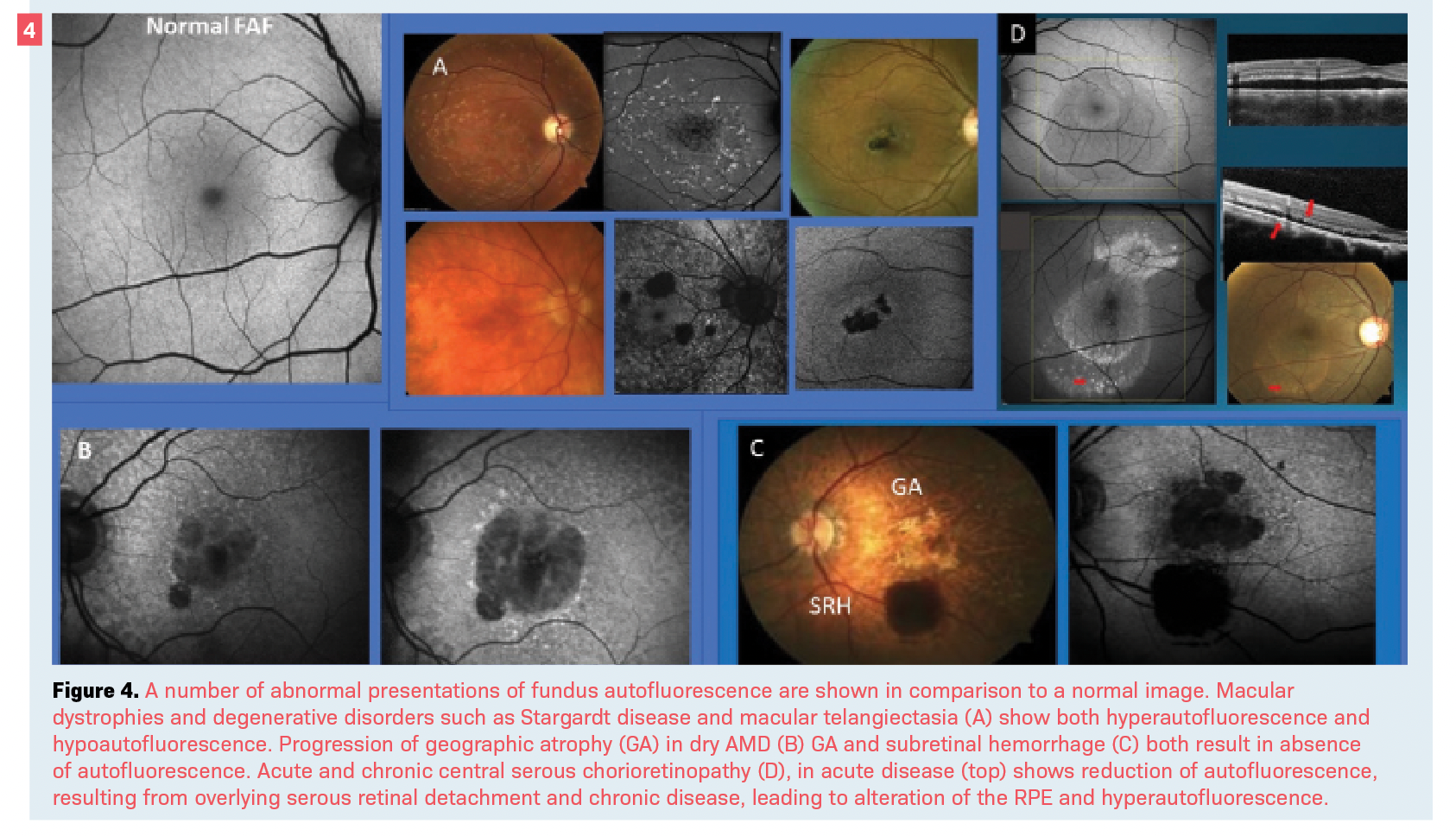
FAF imaging is possible by excitement of lipofuscin as the prominent ocular fluorophore within the retinal pigment epithelium (RPE).9 The accumulation and alteration of lipofuscin by aging and in various disease states, as well as other morphologic changes—such as the presence of certain pigments like hemoglobin in blood—result in either excessive fluorescence (hyperautofluorescence) or the reduction of it (hypoautofluorescence).
When RPE is absent, such as in the case of GA, there is an absolute absence of autofluorescence (Figure 4).9 Although FAF is a valuable diagnostic tool, it will not be a replacement for retinal angiography.
Further, lack of imaging and quantitative standards has been a barrier to broad use of this technology.14
OCT
Since its introduction into the eye care space in the late 1990s, OCT has become one of the most widely used diagnostic tools and has positively influenced our ability in diagnosis and management of posterior segment disease.15
OCT possess features that have led to its success in the eye care world; its principles are like those of ultrasound, but instead of sound and echo it uses a coherent light and reflectance of it to produce high-resolution, cross-sectional images acting as an optical biopsy.16,17
One of OCT’s fortes is in automated intraretinal segmentation.18,19 The ability to recognize and separate each layer of the retina allows for not only accurate morphologic cross-sectional examination or tomographic assessment, but also for various analytical measurements or topographical assessment (Figure 5).
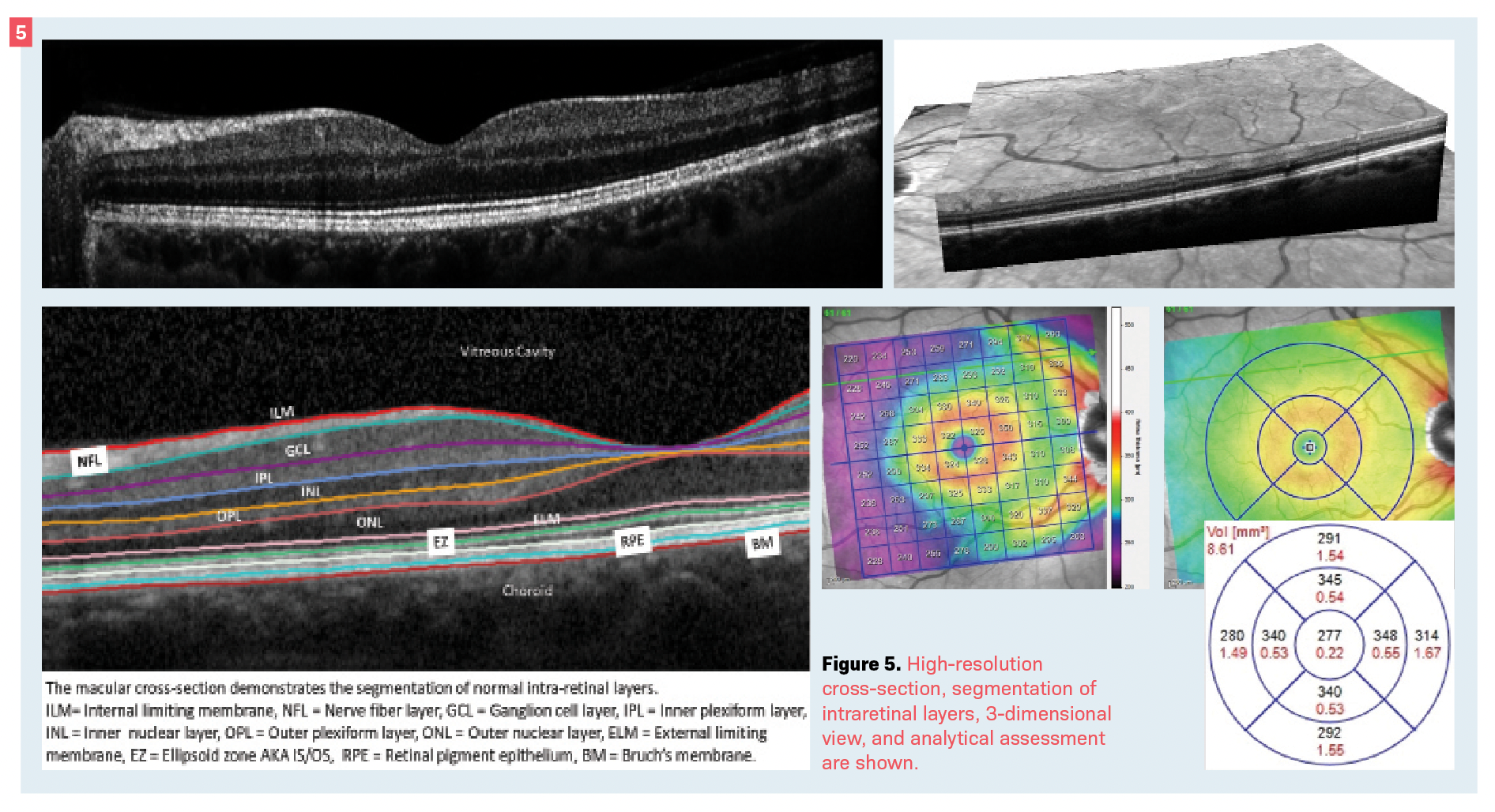
This also allows for 3-dimensional image construction such as en face imaging.20 These features—individually or in combination—can be helpful in the diagnosis of almost any posterior segment disease.
Although most providers utilize OCT mainly for macular and optic nerve disease such as diabetic macular edema (DME), vitreomacular interface diseases (such as macular hole), and glaucoma, this technology can aid in the diagnosis and management of retinal conditions outside the posterior pole (Figure 6).21
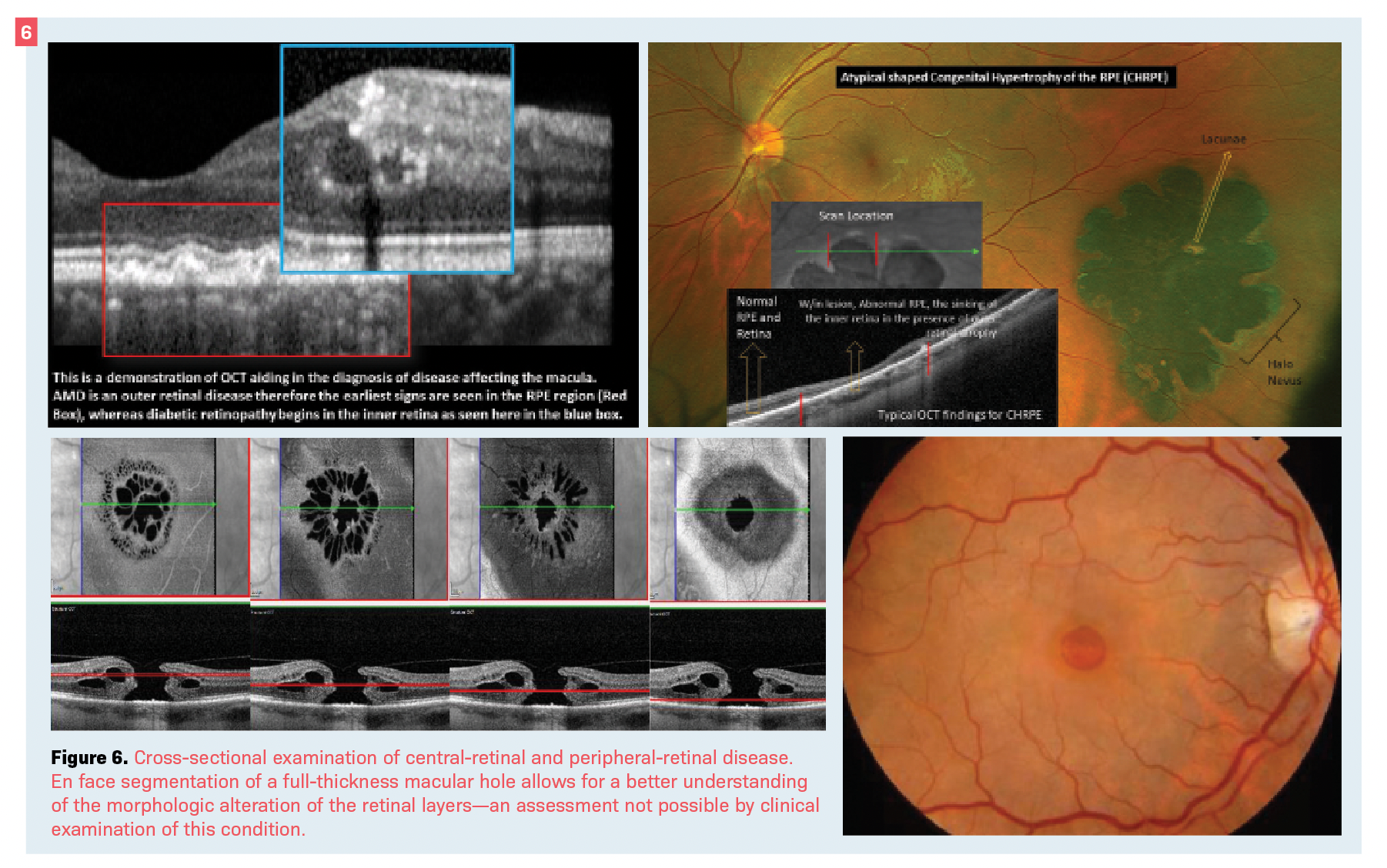
The use of OCT has helped in understanding and managing many peripheral disorders such as pigmentary lesions, white without pressure, lattice degeneration, and peripheral vitreoretinal interface disease like retinal tufts and breaks, as well as differential diagnosis of retinoschisis vs retinal detachment (see Figure 6).22-25
OCT angiography
Retinal segmentation and en face imaging are also the primary developments that have led to the ability to study the vasculature—a technique known as OCT angiography (OCT-A).
Although with no contrast dye utilization, OCT-A is not truly an angiographic, it does have advantages over conventional fluorescein or indocyanine green angiography.
One advantage is the ability to view vascular structure of the retina and optic nerve without the interference of the contrast dye, which changes the tissue observation due to the dynamic nature of the technique.
Another is the ability for OCT-A to evaluate the deep retinal vascular complex (Figure 7).26
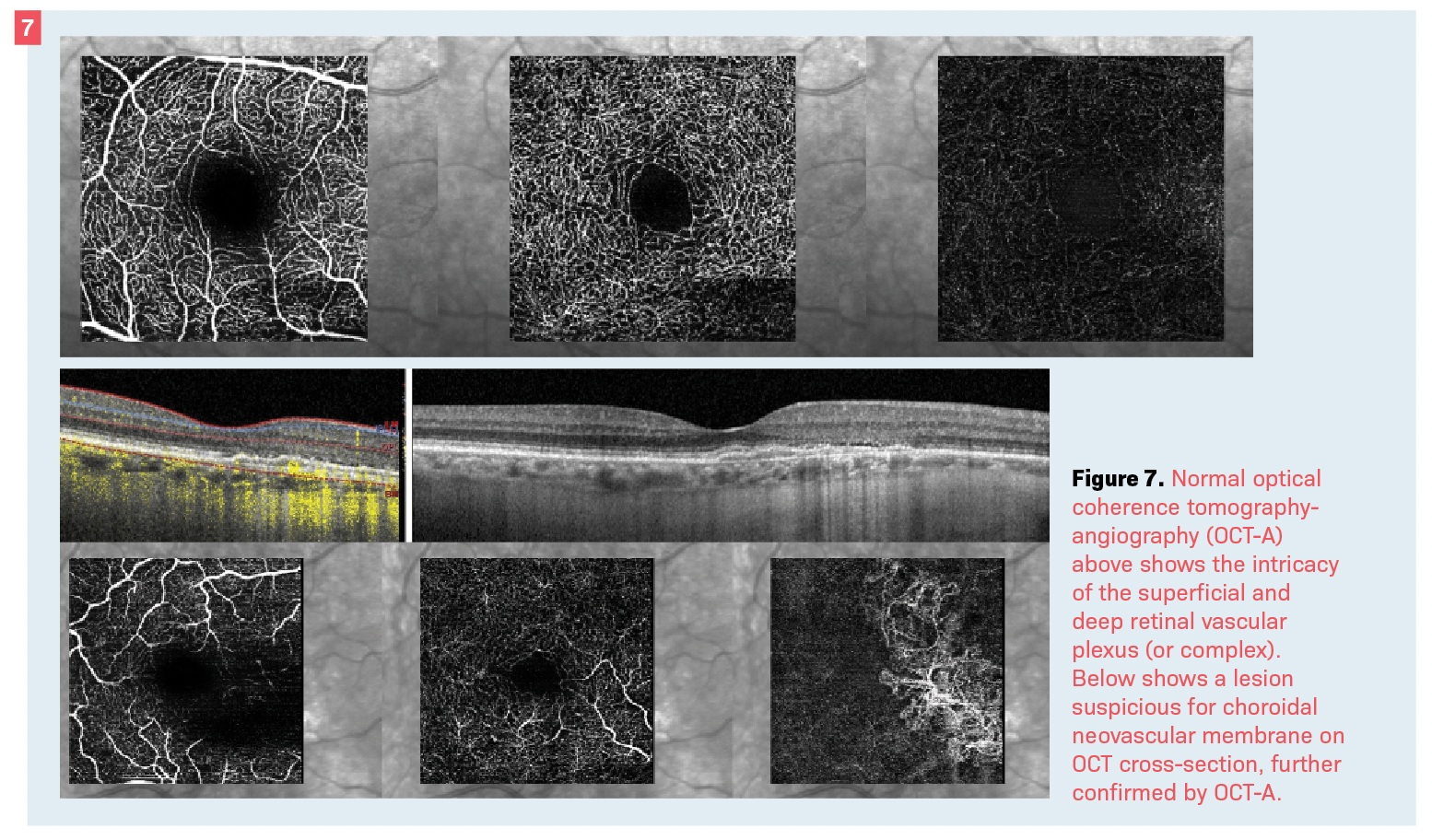
The disadvantage of angiography is also an advantage as compared with OCT-A. The dynamic flow of contrast dye can show “leakage” of fluid—an observation not possible with any other imaging technique (Figure 7).
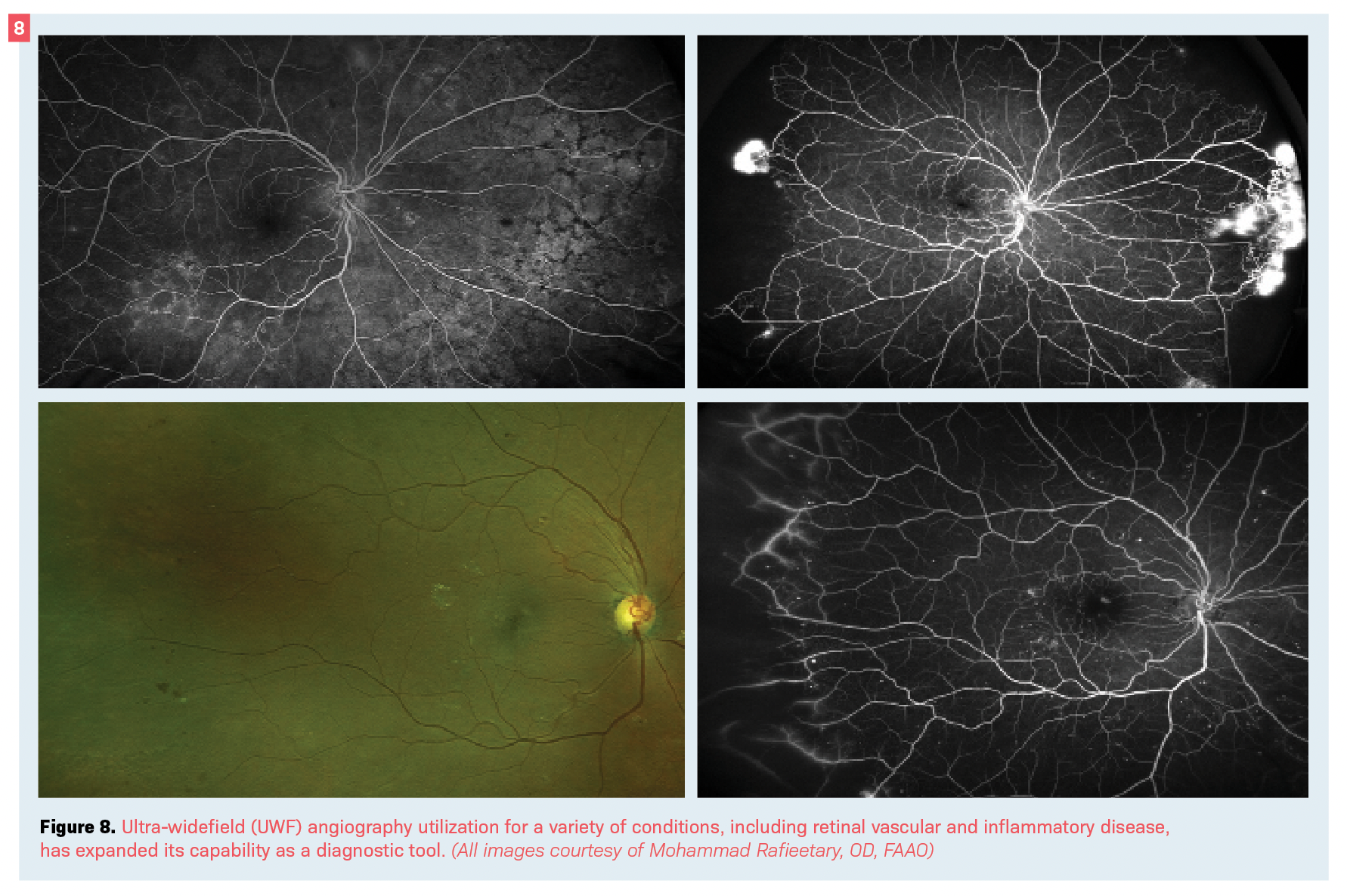
An addition to conventional angiography is the utilization of UWF instruments.
UWF angiography is a valuable tool in evaluating inflammatory, vascular, and many other retinal and chorioretinal disorders (see Figure 8).27,28
Conclusion
The possibility of in vivo examination of the various structures of the ocular tissue is an advantage that many other health care fields do not enjoy.
The transparent tissue of the ocular media allows for development and utilization of various techniques, allowing better diagnosis and improved patient care.
ECPs should stay abreast of all available technologies and utilize those that can be instrumental in aiding with patient care.
References
Bennett TJ. Milestones, rivalries and controversy: the origins of photography and ophthalmic photography part III, the first human fundus photograph. Ophthalmic Photographers’ Society. September 26, 2013. Accessed May 17, 2022. https://www.opsweb.org/blogpost/1033503/171206/Milestones-Rivalries-and-Controversy-Part-III
Terasaki H, Sonoda S, Tomita M, Sakamoto T. Recent advances and clinical application of color scanning laser ophthalmoscope. J Clin Med. 2021;10(4):718. doi:10.3390/jcm10040718
Song JH, Moon KY, Jang S, Moon YR. Comparison of multicolor fundus imaging and colour fundus photography in the evaluation of epiretinal membrane. Acta Ophthalmol. 2019;97(4):533-539. doi:10.1111/aos.13978
Kumar V, Surve A, Kumawat D, et al. Ultra-wide field retinal imaging: a wider clinical perspective. Indian J Ophthalmol. 2021;69(4):824-835. doi:10.4103/ijo.IJO_1403_20
Soliman AZ, Silva PS, Aiello LP, Sun JK. Ultra-wide field retinal imaging in detection, classification, and management of diabetic retinopathy. Semin Ophthalmol. 2012;27(5-6):221-227. doi:10.3109/08820538.2012.708812
Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154(3):549-559.e2. doi:10.1016/j.ajo.2012.03.019
Cleland SC, Domalpally A, Liu Z, et al; Second Carotenoids in Age-Related Eye Disease Study Investigators. Reticular pseudodrusen characteristics and associations in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), an ancillary study of the Women’s Health Initiative. Ophthalmol Retina. 2021;5(8):721-729. doi:10.1016/j.oret.2020.12.019
Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G Jr, Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-575. doi:10.1001/jamaophthalmol.2017.0830
Yung M, Klufas MA, Sarraf D. Clinical application of fundus autofluorescence in retinal disease. Int J Retina Vitreous. 2016;2:12. doi:10.1186/s40942-016-0035-x
Boon CJF, Klevering BJ, Keunen JEE, Hoyng CB, Theelen T. Fundus autofluorescence imaging of retinal dystrophies. Vision Res. 2008;48(26):2569-2577. doi:10.1016/j.visres.2008.01.010
Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42(5):1051-1056.
Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47(8):3531-3538. doi:10.1167/iovs.05-1290
Spaide RF, Klancnik JM Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112(5):825-833. doi:10.1016/j.ophtha.2005.01.003
Frampton GK, Kalita N, Payne L, Colquitt J, Loveman E. Accuracy of fundus autofluorescence imaging for the diagnosis and monitoring of retinal conditions: a systematic review. Health Technol Assess. 2016;20(31):1-108. doi:10.3310/hta20310
Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT1-OCT13. doi:10.1167/iovs.16-19963
Rafieetary MR, Sigler EJ. OCT: interpretation of cross-sectional imaging as an optical biopsy. Advanced Ocular Care. 2013;4(5):27-31.
Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2(1-2):9-25. doi:10.1038/sj.neo.7900071
Mishra A, Wong A, Bizheva K, Clausi DA. Intra-retinal layer segmentation in optical coherence tomography images. Opt Express. 2009;17(26):23719-23728 doi:10.1364/OE.17.023719
Kafieh R, Rabbani H, Kermani S. A review of algorithms for segmentation of optical coherence tomography from retina. J Med Signals Sens. 2013;3(1):45-60.
Heiferman M, Simonett J, Fawzi A. En face OCT imaging in retinal disorders. Retinal Physician. June 1, 2015. Accessed May 17, 2022. https://www.retinalphysician.com/issues/2015/june-2015/en-face-oct-imaging-in-retinal-disorders
Bhende M, Shetty S, Parthasarathy MK, Ramya S. Optical coherence tomography: a guide to interpretation of common macular diseases. Indian J Ophthalmol. 2018;66(1):20-35. doi:10.4103/ijo.IJO_902_17
Choudhry N, Golding J. Taking OCT out to the retinal periphery. Retina Specialist. September 20, 2016. Accessed May 17, 2022. https://www.retina-specialist.com/article/taking-oct-out-to-the-retinal-periphery
Rafieetary M. Navigating the retinal periphery. Review of Optometry. February 15, 2021. Accessed May 17, 2022. https://www.reviewofoptometry.com/article/navigating-the-retinal-periphery
Chu RL, Pannullo NA, Adam CR, Rafieetary MR, Sigler EJ. Morphology of peripheral vitreoretinal interface abnormalities imaged with spectral domain optical coherence tomography. J Ophthalmol. 2019;2019:3839168. doi:10.1155/2019/3839168
Diaz RI, Sigler EJ, Randolph JC, Rafieetary MR, Calzada JI. Spectral domain optical coherence tomography characteristics of white-without-pressure. Retina. 2014;34(5):1020-1021. doi:10.1097/IAE.0000000000000012
Hagag AM, Gao SS, Jia Y, Huang D. Optical coherence tomography angiography: technical principles and clinical applications in ophthalmology. Taiwan J Ophthalmol. 2017;7(3):115-129. doi:10.4103/tjo.tjo_31_17
Rabiolo A, Parravano M, Querques L, et al. Ultra-wide-field fluorescein angiography in diabetic retinopathy: a narrative review. Clin Ophthalmol. 2017;11:803-807. doi:10.2147/OPTH.S133637
Hsia NY, Li YL, Lin CJ, et al. Ultra-widefield angiography in the diagnosis and management of uveitis. Taiwan J Ophthalmol. 2018;8(3):159-163. doi:10.4103/tjo.tjo_115_17

