- Therapeutic Cataract & Refractive
- Lens Technology
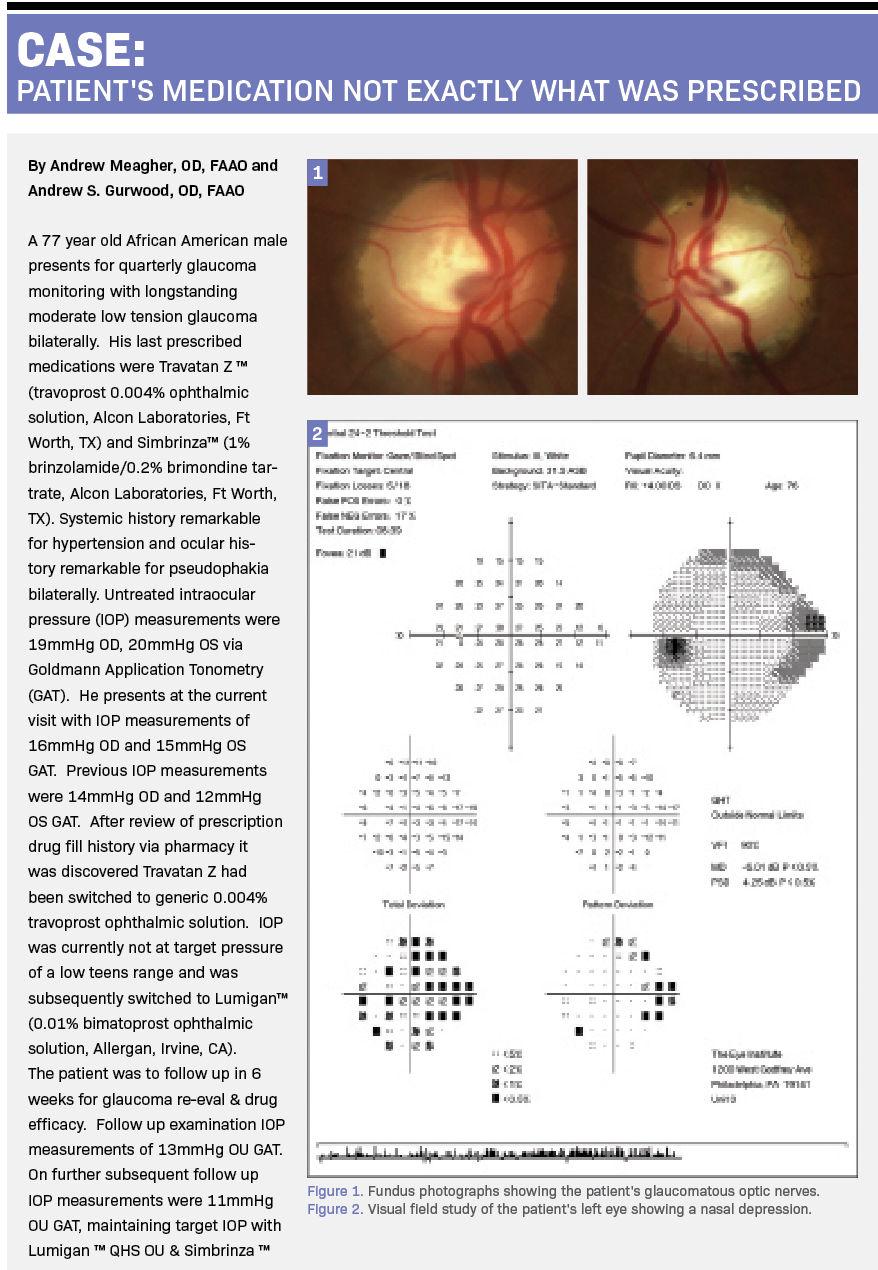
- Glasses
- Ptosis
- AMD
- COVID-19
- DME
- Ocular Surface Disease
- Optic Relief
- Geographic Atrophy
- Cornea
- Conjunctivitis
- LASIK
- Myopia
- Presbyopia
- Allergy
- Nutrition
- Pediatrics
- Retina
- Cataract
- Contact Lenses
- Lid and Lash
- Dry Eye
- Glaucoma
- Refractive Surgery
- Comanagement
- Blepharitis
- OCT
- Patient Care
- Diabetic Eye Disease
- Technology
Generic vs Branded: When RX makes a difference

Treating glaucoma requires management of the only modifiable risk factor: intraocular pressure (IOP). Clinicians have numerous first-line options from which to choose, including the following:
» Five classes of topical medications (prostaglandin analogues, β blockers, α-agonists, carbonic anhydrase inhibitors, and rho kinase inhibitors)
» Four combination formulations (dorzolamide/timolol [Cosopt], Merck & Co, Inc; brimonidine/timolol [Combigan], Merck & Co, Inc; brimonidine/brinzolamide [Simbrinza], Allergan; and latanoprost/netarsudil [Rocklatan], Aerie Pharmaceuticals)
» Four preservative-free options (tafluprost [Zioptan], Akorn Pharmaceuticals; timolol [Timoptic-XE], Merck & Co, Inc; dorzolamide/timolol [Cosopt PF], Merck & Co, Inc; and latanoprost PF [Xelpros], Sun Pharmaceutical Industries Ltd)
» No-drop solutions that include selective laser trabeculoplasty (SLT), cataract extraction with stent placement, and incisional procedures.
Once the data confirm there is conversion to treatable disease and intervention is required, the treating clinician and patient must decide on the modality (drops or no-drop solution) and the agent of intervention (which drop or surgical procedure that will be introduced first).
For patients who cannot instill drops (issues of poor dexterity), afford drops, or obtain drops (loss of ability to operate a computer or drive, do not want the inconvenience of drops, or want to combine cataract surgery with a procedure to lower the IOP), SLT may offer an alternative to topical treatment. SLT is as effective as drops for incident glaucoma cases and offers an outpatient option with results that often last for years1 by which the cellular immune system cleans the trabecular meshwork of debris, permitting more efficient aqueous flow into Schlemm canal. Cataract removal with stent placement creates more room in the middle segment of the eye by creating additional space (increased volume decreases pressure, Boyle’s, Charles’, and Pascal’s laws) with a means of bypassing the funneling of the trabecular meshwork and directly passing aqueous into the Schlemm canal to lower IOP.
When surgery is not desired, topical medications are indicated. Most clinicians recognize choosing a single preparation provides the most pressure lowering/dose with the least inconvenience. This permits the treating physician to understand how much IOP lowering is obtained with that agent. That is also why starting treatment in new cases with a combination preparation is not ideal. Combination therapy should be reserved for new cases that are advanced and fragile, requiring immediate, significant, or extreme IOP lowering, or cases where monotherapy is not achieving an appropriate target pressure (typically around 30% lower than the untreated IOP).2 In this way, the combination drop combines the symbiotic IOP-lowering effects of 2 drops into 1 convenient system.
The prostaglandin class offers IOP lowering without the risk of central nervous system or systemic adverse effects.3 The algorithm after this varies based on the clinician’s experience and style and may include switching drops, adding more drops, considering adding SLT, and referral for other surgical solutions.4
Starting a patient on drops becomes complicated when patients believe the drops are uncomfortable to instill, make their eye red, claim they affect vision, or are too expensive. This makes topical prescribing not only an art but also something that should not be handled casually. The clinician choice is based on experience for efficacy and comfort, making the choice brand specific. The choice of one medication over another is based upon knowledge of the manufacturer, an understanding of the chemistry, and observation of success.
Not all drops are the same. Although generic substitutions can offer a cost advantage, they are not always equivalent to their branded counterpart. Understanding the intricacies of generic manufacturing provides clinicians with the knowledge to either permit a responsible substitution or insist on branded use.
The OHTS (NCT00000125) and EMGT (NCT00000132) showed that every 1-mm Hg increase from the baseline IOP created a 10% to 13% increase in risk for conversion to the treatable form of the disease.5,6 The EMGT also concluded each initial 1-mm Hg reduction in IOP at the 3-month follow-up created an 8% decrease in risk of progression.6 This makes the initial IOP reduction a serious consideration, in that every mm Hg of IOP reduction may be the difference between substantial visual loss and maintaining current visual status.5,6 The initial prescribing choices should not be taken lightly. In fact, most clinicians use branded sample medication to determine whether their decision is acceptable to move forward, with a 30% reduction from the untreated IOP considered a proper initial target. It should not be assumed that a generic medication can deliver the same IOP-lowering effect as its branded counterpart.
Generic drugs background
The US Food and Drug Administration (FDA) defines generic drugs as medications created by combining the same ingredients as an existing, approved, brand-name drug. The medication must work using the same dosage and provide the same safety profile, strength of action, route of administration, quality or efficacy, and performance characteristics.7-9 Branded medications become available for commercial manufacture when their patent rights expire, often after 7 to 10 years of exclusive production rights. When another manufacturer chooses to produce a particular medication whose patent protection is expiring, it takes some time to create the physical plant and meet all guidelines. Considering this, generics are often released between 7 and 10 years after a branded medication becomes commercially available.10
The standards to produce generic medication, specifically ophthalmic agents, are far less stringent than those that branded drugs had to pass for the initial FDA approvals. The government assumes the new manufacturer is simply assembling the components of a branded medication that has already been tested and combining those ingredients in a similar fashion.8,11 However, this analogy applies: When you eat at great-grandma’s house, her infamous pasta sauce is fabulous. You and your relatives ask her for the recipe, and great-grandma is flattered to share the recipe with everyone who wants it. Unfortunately, even though the entire family uses the exact same ingredients, none captures the same flavor as when great-grandma makes it.
Generic drugs make up almost 90% of the pharmaceutical market in the US. For some perspective, in 1984, roughly one-fifth of the drug market was generic, and by 1996 that level had risen to just over 40%.12 The publicized advantage of generic drug manufacturing is cost savings to the patient (generic drug manufacturing fixed costs are minimized because the new commercial manufacturer does not have the extreme expenditures in research, development, or testing). Between 2007 and 2016, the US approximated savings of $1.68 trillion.13
A personal informal investigation recently identified patients using travoprost (Travatan Z; Novartis) who were switched to generic travoprost over the course of the COVID-19 pandemic. During this time, prescribing physicians had less control over the medications dispensed by apothecaries. Postpandemic ophthalmic examination found that patients who were once well controlled for years prepandemic were returning with IOPs consistently higher than before. The issue was initially considered idiopathic. However, as more and more cases were uncovered, a trend emerged. An investigation led to 4 questions posed to patients to narrow down what could have been a multifactorial problem: (1) Was there an issue with adherence during home isolation? (2) Did an interruption in medication refills cause a lapse in treatment? (3) Was tonometry accuracy altered because of the use of disposable tonometer tips? (4) Were the drops properly and consistently used during the pandemic period?
The common thread was that the pharmacies had changed their branded topical medications to generic medications without the consent of the prescribing doctor or notification of the patient. Before the pandemic, unless a patient brought their medication in, discovering that a branded medication was replaced with a generic was rare. Even when the IOP was a few points higher than the previous measurement reading—because it was not immediately pathological requiring a switch or addition—the patient was allowed to continue with the medication they were using. When the readings increased more or never returned to the original levels or new prescriptions were written for brand-specific agents, the discovery was made. The remedy was not complicated—switch back to the brand-name medication and recheck the IOP to see the result. The consistently lower IOPs justified prescribing brand-specific topical medications.
Insurance companies are in the business of generating profits, which they do partly by minimizing expenditures. When drug companies invest multimillions of dollars in research and development, trials, and marketing to bring a new medication to the market, those costs require recoupment. As such, branded medications cost more than the generic counterparts, which is why insurance companies are reluctant to cover them. Branded medications gain insurance support by being placed onto their formulary. The concerned prescriber can avoid delays and inconvenience but determining which medications are covered and picking from that list.
The generic drug approval process
The FDA’s role in generic drug manufacturing includes rigorous reviews to ensure high-standard drug manufacturing facility inspections and drug safety monitoring.8,14 Although the active drug ingredients are required to be the same, many of the other components in the vehicle have latitude. These components include solution properties, such as viscosity, drug excipients, such as particulate matter, drop size related to viscosity, and surface tension. Further, the chemical design of the molecule may be different. A 2012 article reviewed differences in North American branded vs generic bottle design, drop viscosity, surface tension, and volume, and found substantial differences.15
Manufacturers of generic medications do not have to repeat animal or clinical research completed by the original manufacturer. The head-to-head efficacy studies (generic vs branded) that are performed are short (typically 3 months or less) and do not view functional visual loss as the end point. This means the drug passes even if a patient got functionally worse, as long as the IOP remained within the appropriate range compared with the brand.16 There are no specific authorities that regulate generic medications. A meta-analysis or systematic review for efficacy compared with branded medications is not required.16,17 The FDA does require a generic drug to demonstrate similar bioequivalence to the branded product. A medication is considered bioequivalent if it has a comparable absorption profile of its active ingredients via blood or body fluid measurements. Unfortunately, measuring this is challenging for ophthalmic drugs (drops).8,18-20 The FDA provides an exemption for products whose bioequivalence studies cannot be completed; these are often locally acting drugs, such as eye drops. A locally effective medication is exempt when there is no process by which measurable quantities of any product can be found in the bloodstream, and any measurable levels that are found are not deemed to be relating to actual drug efficacy.21 An example of the large and disparate levels of active medication between generic and branded medication was seen in a 2015 study in which latanoprost generic medications were discovered to dispense concentrations that varied from 90% to 330% of the labeled amount vs a consistent 97% in the branded compound.22 The point is, one is never sure what is consistently being dispensed.
Although the active ingredient molecule is required to be the same, a drug’s temperature stability can also lead to deleterious effects. Variations in particulate matter, primarily thought to be due to contaminants or active ingredient precipitates, have also been shown to lead to faster chemical degradation when exposed to temperatures of 77 °F and higher. The movement and packaging of generic drugs do not mandate temperature regulation, which provides more sources for disparity. Because latanoprost is unstable at higher temperatures, patients should be advised to refrigerate it after opening. Carrying it in a pocket or pocketbook or leaving it exposed to sunlight risks deactivating the medication and must be discouraged.
The FDA oversees the active ingredients in a medication, the other inactive ingredients (excipients) are monitored but allowed to vary. Excipients primarily include buffers and thickening agents. These inert ingredients are not pharmacologically active but can affect bioavailability and drug stability and negatively potentiate efficacy. They must be quantitatively similar and qualitatively identical to the branded medication (quantitatively means similar in viscosity, surface tension, pH, and density).11,19,23,24
Lower levels of the preservative benzalkonium chloride found in generic latanoprost have proposed to be a factor in faster active ingredient degradation.25,26
Titcomb examined differences between branded and generic latanoprost and found a lower pH in the branded agent.27 Narayanaswamy et al proposed that higher pH levels in generic latanoprost formulations were the primary reason for reduced efficacy.28 Kahook et al evaluated drug composition via mass spectroscopy and found marked differences in both active ingredients and excipients when the branded agents were compared with the generics. Although the FDA requires drugs to be within 10% of the composition of the reference drug, these data suggest this element of the manufacturing process is not heavily regulated.25
All liquids possess surface tension (ST), a lower level of which results in a smaller drop. It was found that generics had anywhere from 18% to 25% lower ST compared with branded counterparts. This accounts for an average drop volume difference. Overall, a larger drop volume was dispensed in generic preparations.15
Viscosity also plays a role in drop volume and overall drug delivery. Of the medications that used the gel-based xanthan gum polymer, lacrimal drainage decreased, resulting in greater bioavailability by allowing the vehicle to create a temporary plugging effect in the punctum.29
Generic Timoptic-XE was found to have xanthan gum as its vehicle, whereas gellan gum was used in the branded medication. The gellan gum compound provides significantly improved IOP lowering 8 hours after instillation vs the xanthan gum variant.30,31
Other unregulated issues pertaining to differences in equivalencies included bottle design, physiochemical properties of the solution, and variance in drop volume. In generics vs branded medications, these are issues, especially considering the idiosyncrasies of patient bottle handling and drop dispensing.15,32
No regulations currently exist for bottle design between branded and generic medicines. Drop delivery, drop volume, and bottle performance coupled with dexterity are legitimate concerns. Generic bottle designs affect drop administration via orifice opening and feasibility to squeeze the drop out. Kolko et al found much less effort necessary to squeeze and dispense a drop of branded latanoprost vs generic latanoprost.15,24,32
Another inconsistency includes suppliers and supply chains. Pharmacies use an armament of different generic retailers where sales are dictated by market costs and medication supplies with little acknowledgement of quality controls. This can lead to variations within the supplies of the generic drugs themselves.
Conclusion
The use of generic medications to save the medical economy is reasonable. With longer life span in the setting of increasing costs, generic preparations might offer sufficient savings to present a reasonable argument for their support.
Unfortunately, there are enough pitfalls to offset the advantages, and when topical treatments fail and surgical solutions are required that necessitate more office visits and complications, one questions whether anything was really saved. Generic topical medications will not disappear from the marketplace.
However, clinicians should be informed when they are substituted for brand medications so patients can be monitored closely for possible treatment failure.

References
1. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (LiGHT) trial. A multicentre, randomised controlled trial: design and methodology. Br J Ophthalmol. 2018;102(5):593-598. Published correction appears in Br J Ophthalmol. 2021;105(2):e1.
2. AGIS Investigators: The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-440. doi:10.1016/s0002-9394(00)00538-9
3. Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53(suppl 1):S107-S120. doi:10.1016/j.survophthal.2008.08.010
4. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2018:1118.
5. Kass MA, Heuer DK, Higginbotham EJ, eta l. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-830. doi:10.1001/archopt.120.6.701
6. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268-1279. doi:10.1001/archopt.120.10.1268
7. Generic drugs: questions & answers. FDA. Updated September 9, 2010. Accessed April 22, 2021. www.fda.gov/drugs/resourcesforyou/consumers/questionsanswers/ucm100100.htm
8. Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003;25(11):2875-2890. doi:10.1016/s0149-2918(03)80340-5
9. Cantor LB. Generic ophthalmic medications: as good as a Xerox? Medscape. Accessed April 22, 2021. https://www.medscape.org/viewarticle/583866
10. Generic and hybrid medicines. European Medicines Agency. Accessed July 29, 2021.https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/generic-hybrid-medicines
11. Fiscella RG, Gaynes BI, Jensen M. Equivalence of generic and brand-name ophthalmic products. Am J Health Syst Pharm.2001;58(7):616-617. doi:10.1093/ajhp/58.7.616
12. Cook A, Acton JP, Schwartz E. How increased competition from generic drugs has affected prices and returns in the pharmaceutical industry. The Congress of the United States Congressional Budget Office. July 1998. Accessed October 1, 2021. https://www.cbo.gov/sites/default/files/105th-congress-1997-1998/reports/pharm.pdf
13. Generic drug access & savings in the US report. Association for Accessible Medicines. Accessed April 22, 2021. https://accessiblemeds.org/sites/default/files/2018_aam_generic_drug_access_and_savings_report.pdf
14. Cantor LB. Ophthalmic generic drug approval process: implications for efficacy and safety. J Glaucoma. 1997;6(5):344-349. doi:10.1097.00061198-199710000-00011
15. Mammo ZN, Flanagan JG, James DF, Trope GE. Generic versus brand-name North American topical glaucoma drops. Can J Ophthalmol. 2012;47(1):55-61. doi:10.1016/j.jcjo.2011.12.004
16. Tatham AJ. The use of generic medications for glaucoma. J Ophthalmol.2020;2020:1651265. doi:10.1155/2020/1651265
17. WHOexpert committee on specifications for pharmaceutical preparations. World Health Organization. 2016. Accessed October 1, 2021. https://www.who.int/medicines/publications/pharmprep/WHO_TRS_996_web.pdf
18. The safety and effectiveness of generic drugs. Health Canada. Updated April 2021. Accessed April 22, 2021. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/hl-vs/alt_formats/pdf/iyh-vsv/med/med-gen-eng.pdf
19. Cantor L. Generics: not all drugs are created equal. Rev Ophthalmol. 2002;9:72.
20. Guideline on the Investigation of Bioequivalence. European Medicines Agency. January 20, 2010. Accessed April 22, 2021. https://www.ema.europa.eu/en/documents/ scientific-guideline/guideline-investigation-bioequivalence- rev1_en.pdf
21. Diagourtas A, Kagelaris K, Oikonomakis K, Droulias A, Kokolakis N, Papaconstantinou D. Prospective study comparing Xalatan eye drops and two similar generics as to the efficacy and safety profile. Eur J Ophthalmol. 2018;28(4):378-384. doi:10.1177/1120672117747030
22. Velpandian T, Kotnala A, Halder N, Ravi AK, Archunan V, Sihota r.Stability of latanoprost in generic formulations using controlled degradation and patient usage simulation studies. Curr Eye Res. 2015;40(6):561-571. doi:10.3109/02713683.2014.939763
23. Aref AA.Generic drugs for the treatment of ocular conditions: changing the treatment landscape. Exp Rev Clin Pharmacol. 2014;7(5):551-553. doi:10.1586/17512433.2014.928197
24. Kolko M, Koch Jensen P. The physical properties of generic latanoprost ophthalmic solutions are not identical. Acta Ophthalmol. 2017;95(4):370-373. doi:10.1111/aos.13355
25. Kahook MY, Fechtner RD, Katz LJ, Noecker RJ, Ammar DA.A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37(2):101-108. doi:10.3109/02713683.2011.631722
26. Leitritz MA, Lipp HP, Voykov B, Ziemssen F. Original preparations versus generics--latanoprost: how similar is different? Ophthalmologe. 2015;112(2):127-139. doi:10.1007/s00347-014-3097-x
27. Titcomb L.Help ensure that the change to generic latanoprost is free of problems. The Pharmaceutical Journal. 2012;288:709.
28. Narayanaswamy A, Neog A, Baskaran M, et al. A randomized, crossover, open label pilot study to evaluate the efficacy and safety of Xalatan in comparison with generic latanoprost in subjects with primary open angle glaucoma or ocular hypertension. Indian J Ophthalmol. 2007;55(2):127-131. doi:10.4103/0301-4738.30707
29. Greaves JL, Wilson CG, Rozier A, Grove J, Plazonnet B. Scintigraphic assessment of an ophthalmic gelling vehicle in man and rabbit. Curr Eye Res. 1990;9(5):415-420. doi:10.3109/02713689008999606
30. Schenker HI, Silver LH. Long-term intraocular pressure-lowering efficacy and safety of timolol maleate gel-forming solution 0.5% compared with Timoptic XE 0.5% in a 12-month study. Am J Ophthalmol. 2000;130(2):145-150. doi:10.1016/s0002-9394(00)00458-x
31. Stewart WC, Sharpe ED, Stewart JA, Hott CE. The safety and efficacy of timolol 0.5% in xanthan gum versus timolol gel forming solution 0.5%. Curr Eye Res. 2002;24(5):387-391. doi:10.1076/ceyr.24.5.387.8516
32. Van Santvliet L, Ludwig A. Determinants of eye drop size. Surv Ophthalmol. 2004;49(2):197-213. doi:10.1016/jsurvophthal.2003.12.009

