How to diagnose a swollen optic nerve
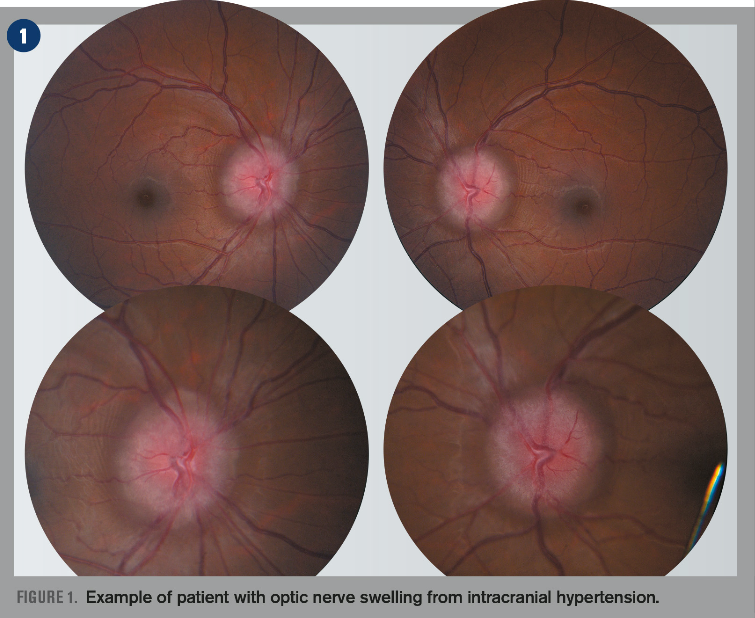

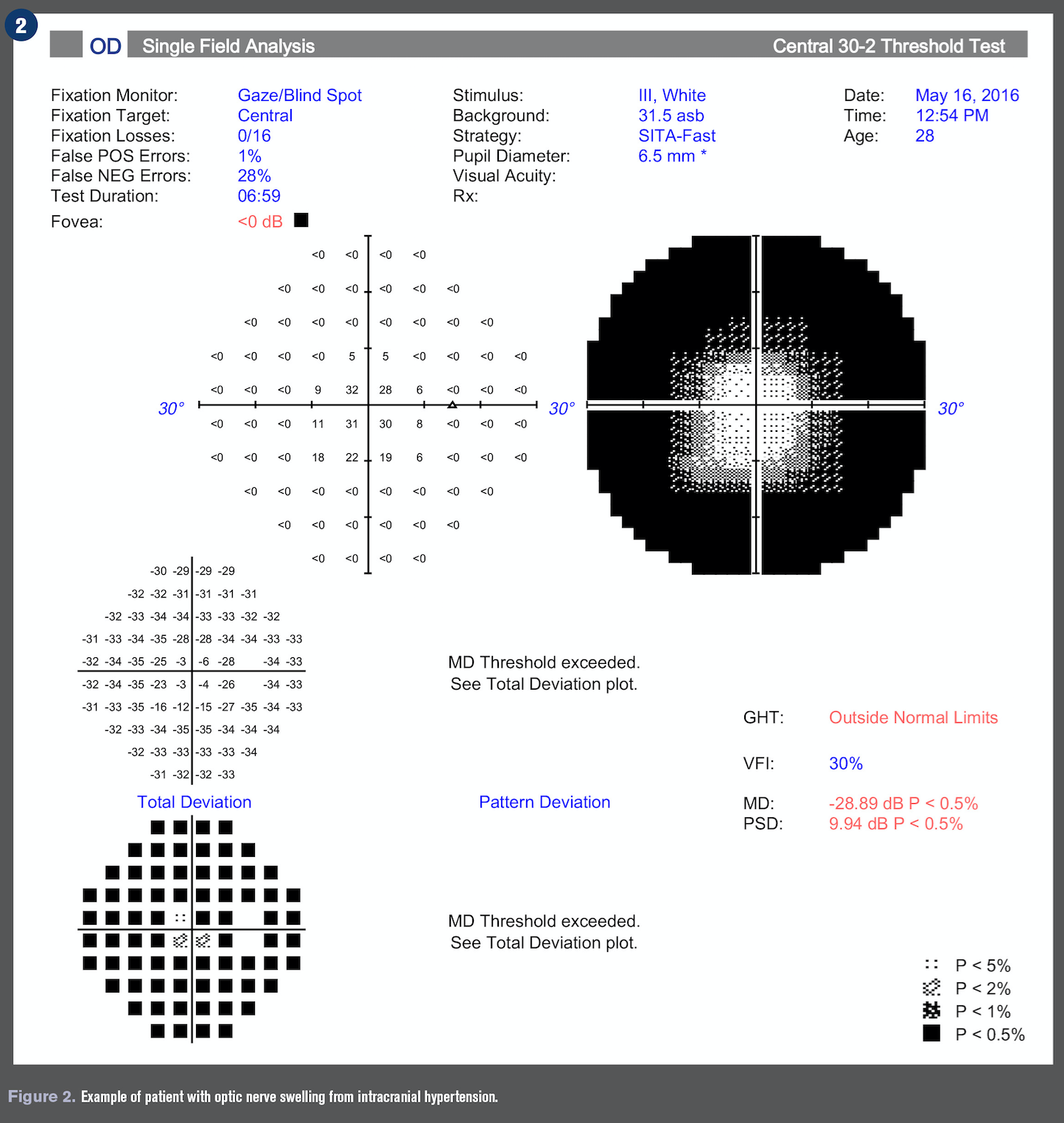
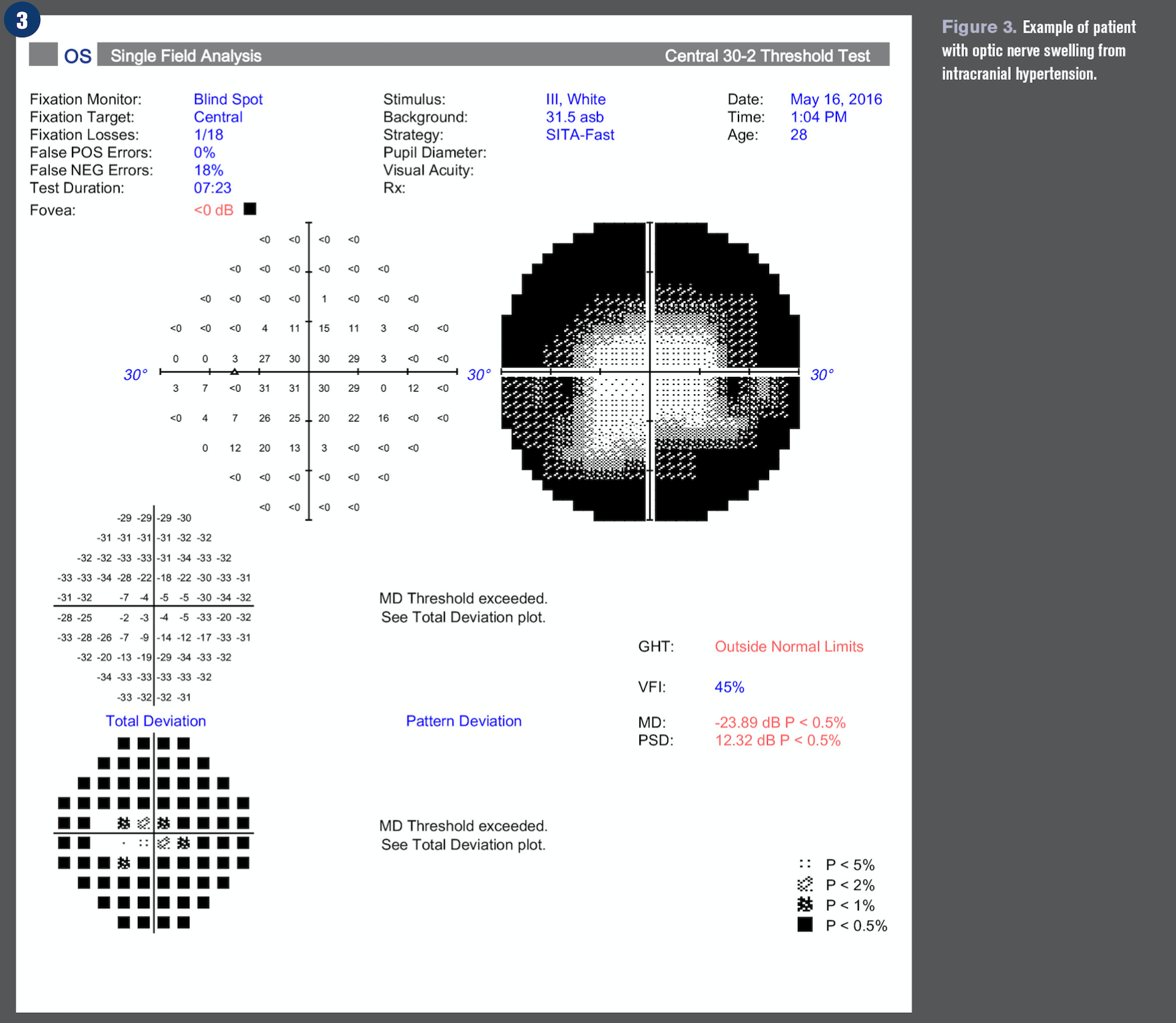
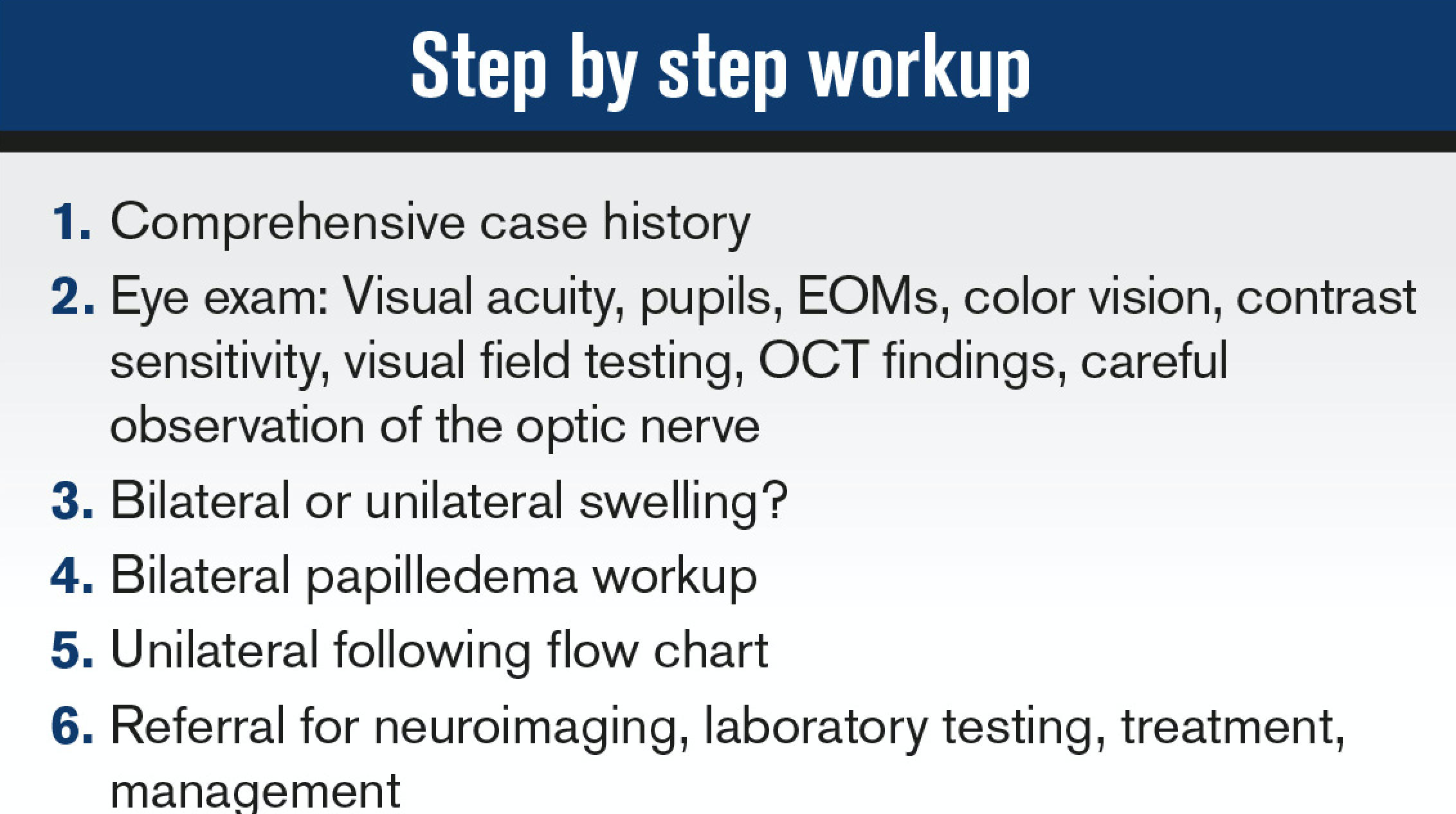
Optic nerve swelling or edema can be an intimidating finding in the primary eyecare clinic. The diagnosis of a swollen optic nerve is based on clinical diagnosis, but the importance of a comprehensive case history cannot be overstated.
Ocular history
During the history, many important questions should be asked to help determine the etiology of the optic nerve edema. The clinician should begin by questioning if the condition is a one or two eyeball problem.
Bilateral optic nerve edema is a medical emergency.
Related: Optic nerve swelling a sign of cat-scratch neruoretinitis
The onset and severity of vision loss and symptoms are important clues to consider. Rapid onset is characteristic of ischemic optic neuropathy, inflammatory and traumatic causes, and optic neuritis.
A gradual onset over time is typical of compressive, hereditary, toxic, and nutritional causes.
Another important symptom: pain associated with eye movements. If present, it is hallmark differential for optic neuritis.
Systemic history
A detailed history of systemic health is important. Diabetes, hypertension, high cholesterol, and a history for being treated for or having malignancy or autoimmune disease is critical to understand potential risks associated with optic nerve edema.
Current and previous medications can be clues because many can be directly or indirectly toxic to the optic nerve. These drugs include tetracycline, cyclosporine, methotrexate, ethambutol, amiodarone, alcohol, and tobacco, to name a few.
Finally, reviewing the patient’s general health, including weight, eating habits, and social activities such as drinking, smoking, and illegal drugs will help provide an overall health picture, knowing that toxic and nutritional causes are in the differential diagnosis paradigm.
Clinical exam
The clinical eye exam is the second step of process. With any optic nerve disease, ODs should pay close attention to several exam elements.
Important exam elements include:
• Subjective and objective visual acuity
• Pupils
• Extra-ocular muscles
• Color vision
• Contrast sensitivity
• Visual field testing
• Anterior and posterior chamber observation
• Optic nerve observation
Visual acuity can be normal or impaired, and subjectively a patient may complain of blurry letters or shadows around letters and an overall decrease in clarity.
Pupil testing is extremely important in assessing optic nerve disease. A relative afferent pupillary defect (RAPD) can be detected performing the swinging penlight pupil test conducted in a dark room to assess full amplitude of pupillary response.
Color vision and color desaturation testing can be performed with Ishihara color plates and red-capped bottle. Dyschromatopsia, or the ability to perceive colors as abnormal, is a sensitive indicator of optic nerve disease.
Related: Optic nerve exam key to assessment
During the dilated fundus exam, the clinician should look for evidence of cells in the anterior chamber and vitreous. This would indicate that the disc swelling is secondary to a uveitic process.
When assessing the optic nerve, stereoscopic views of the disc provide the quality and detail needed to determine disc edema from elevation. The optic nerve will appear elevated and hyperemic with blurred margins obscuring peripapillary vessels as they leave the disc.
It is important to assess the spontaneous venous pulsation (20 percent of normal population)-it will not be seen if swelling is present.
Another clinical sign to look for is buckling or retinal folds of the temporal aspect of the disc, also known as Paton’s lines.
If optic nerve edema is suspected, the standard method of grading disc edema by ophthalmoscopy is the Modified Frisén Scale. It is an ordinal scale with subjective evaluation and grades ranging from 0 (no edema) to 5 (severe degree of edema) with characteristic findings for each grade.1
Additional testing crucial to accurately diagnosing optic nerve swelling are visual field (VF) testing and optical coherence tomography (OCT).2
Related: 6 OCT pitfalls to avoid
VF testing can be confrontation, kinetic (Goldmann), or automated static perimetry (Humphrey). VF defects take several patterns: central, arcuate, altitudinal, and generalized. Specific patterns can help the clinician correlate to specific diagnoses.
OCT uses light to provide images to the micron level and is an objective, noninvasive alternative to analyze the optic nerve head and quantify the status of the retinal nerve fiber layer (RNFL). Elevation of the RNFL is compared to age-related normative values to help confirm the already visualized ophthalmoscopy Frisén Scale findings.1
Bilateral optic nerve swellling
Papilledema is acquired bilateral optic nerve swelling due to increased intracranial pressure secondary to increased cerebral spinal fluid (CSF), which has specific etiologic implications. Papilledema is a true ocular emergency because it can occur from structural space occupying lesions such as large tumors, hydrocephalus, vascular abnormalities, cerebral venous sinus thrombosis, and arteriovenous fistulae.3,4
Due to these pathologies, it is imperative to begin the process of neuroimaging, including magnetic resonance imaging (MRI) with contrast of the brain, orbit, and optic nerve, and magnetic resonance venography (MRV) of the brain.
A referral to neuroophthalmology is warranted to evaluate and manage findings from the results of neuroimaging. If neuroimaging is negative, it is important to follow up with lumbar puncture to measure opening pressure and biochemistry, microbiology, and cytology of the CSF.
Note papilledema in the pediatric population may include but not limited to Guillain-Barre syndrome, spina bifida, hydrocephalus, trauma/subdural hemorrhage, and meningitis.5,6
Related: OCT in pediatric eye disease
After exclusion of potentially life-threatening conditions and a normal CSF, determine if the opening pressure on lumbar puncture is elevated (>20cm H20).
It is also important to note that the modified Dandy criteria are still not met. This diagnostic criteria mandate identifying other potential causes is necessary-idiopathic intracranial hypertension (IIH) is a diagnosis of exclusion once all other potential causes have been ruled out.â·
The most common presenting symptoms are headache, occurring in more than 90 percent of patients, as well as pulsatile tinnitus, photopsia, and retrobulbar pain.8,9 First-line treatment for IIH should concentrate on weight loss and management with a neuro ophthalmologist to lower CSF with an oral carbonic anhydrase inhibitor such as acetazolamide combined with a low-sodium diet as described in the IIHTT.10
In patients with sudden and severe papilledema who may be at risk for irreversible vision loss, surgical intervention such as optic nerve sheath fenestration and cerebrospinal fluid shunting may be urgently needed.11 See Figures 1-4 for an example of a patient with optic nerve swelling from IIH.
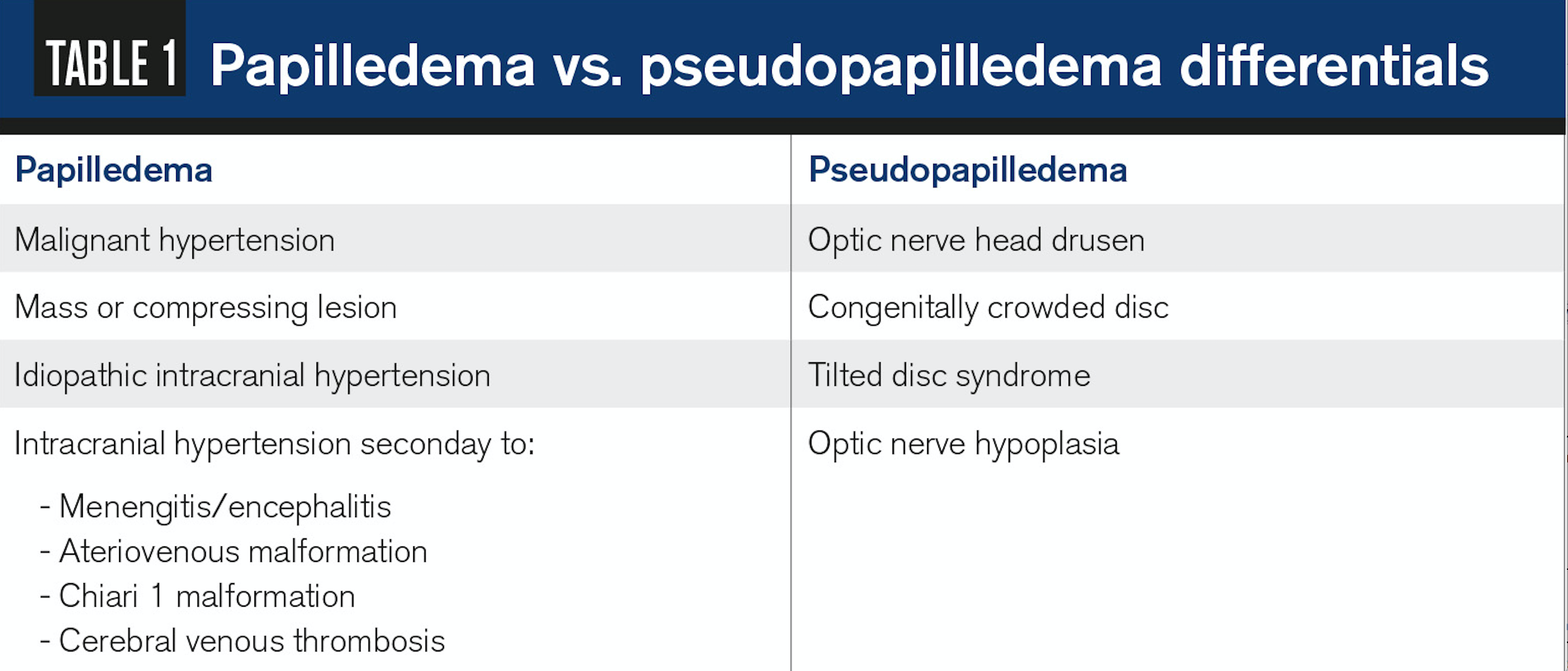
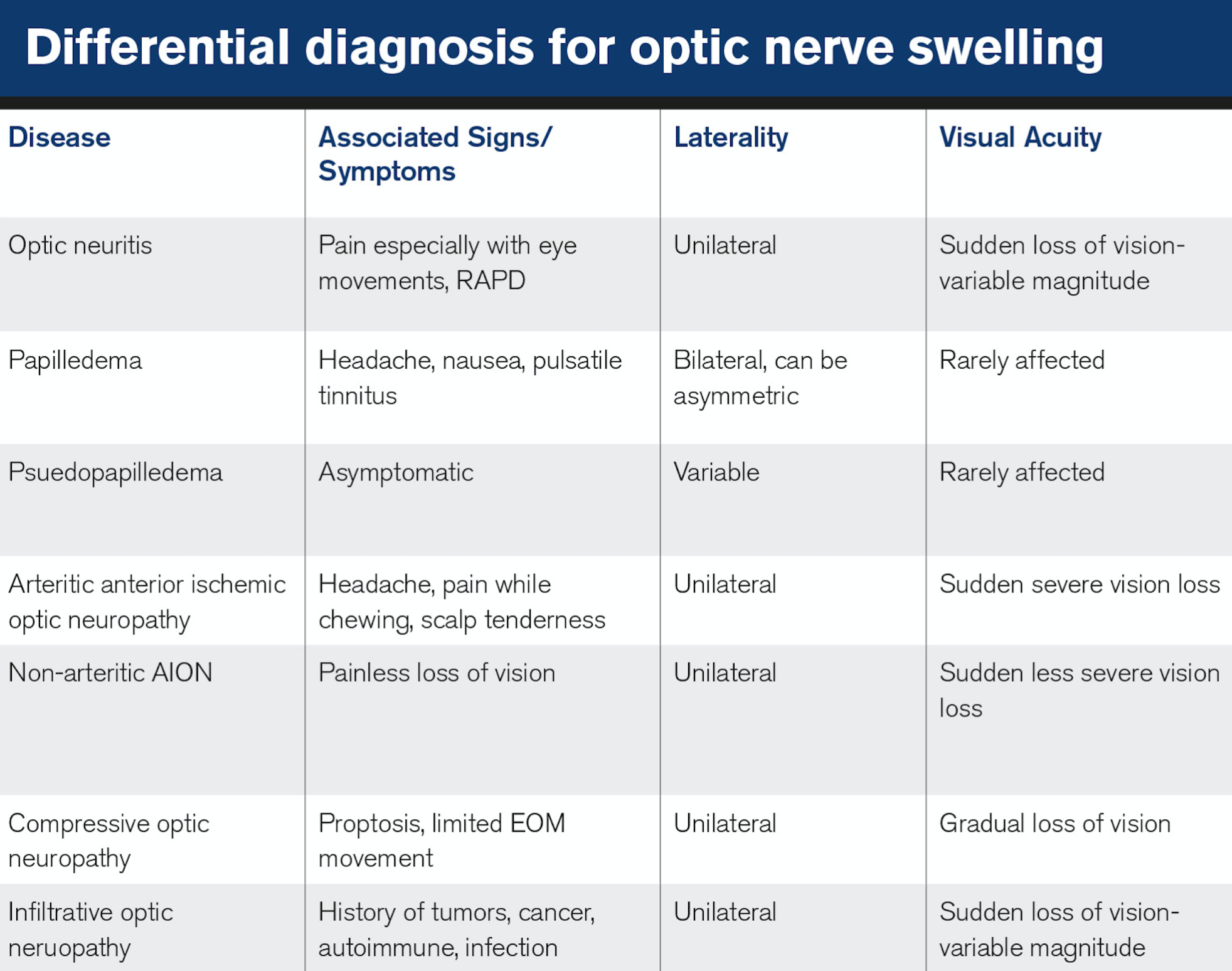
It is key to rule out pseudo papilledema, a benign elevation of the optic nerve head with an underlying anomalous condition (see Table 1). These anomalies include optic nerve head drusen (ONHD), congenital crowded discs, and malinserted discs.
ONHD is the most common cause, accounting for 75 percent of clinically diagnostic disc anomalies.12 The scleral canal and optic disc of eyes with drusen are much smaller than average, thus showing elevated appearance.
Related: The case of the blurred disc margins
OCT can aid in differentiating disc drusen from edema. Disc edema has a smooth internal disc contour and demonstrates a V-shaped hypo reflective space between sensory retina and RPE compared to the lumpy appearance found with disc drusen.13
Congenital crowded discs are the result of a normal number of retinal axons passing through a small posterior scleral foramen with the resulting appearance of a densely packed optic nerve head as axons exit the globe. A malinserted disc is due to oblique insertion of the nerve to the globe; primarily the nasal portion is elevated with the temporal portion depressed, giving it a swollen-like appearance.14
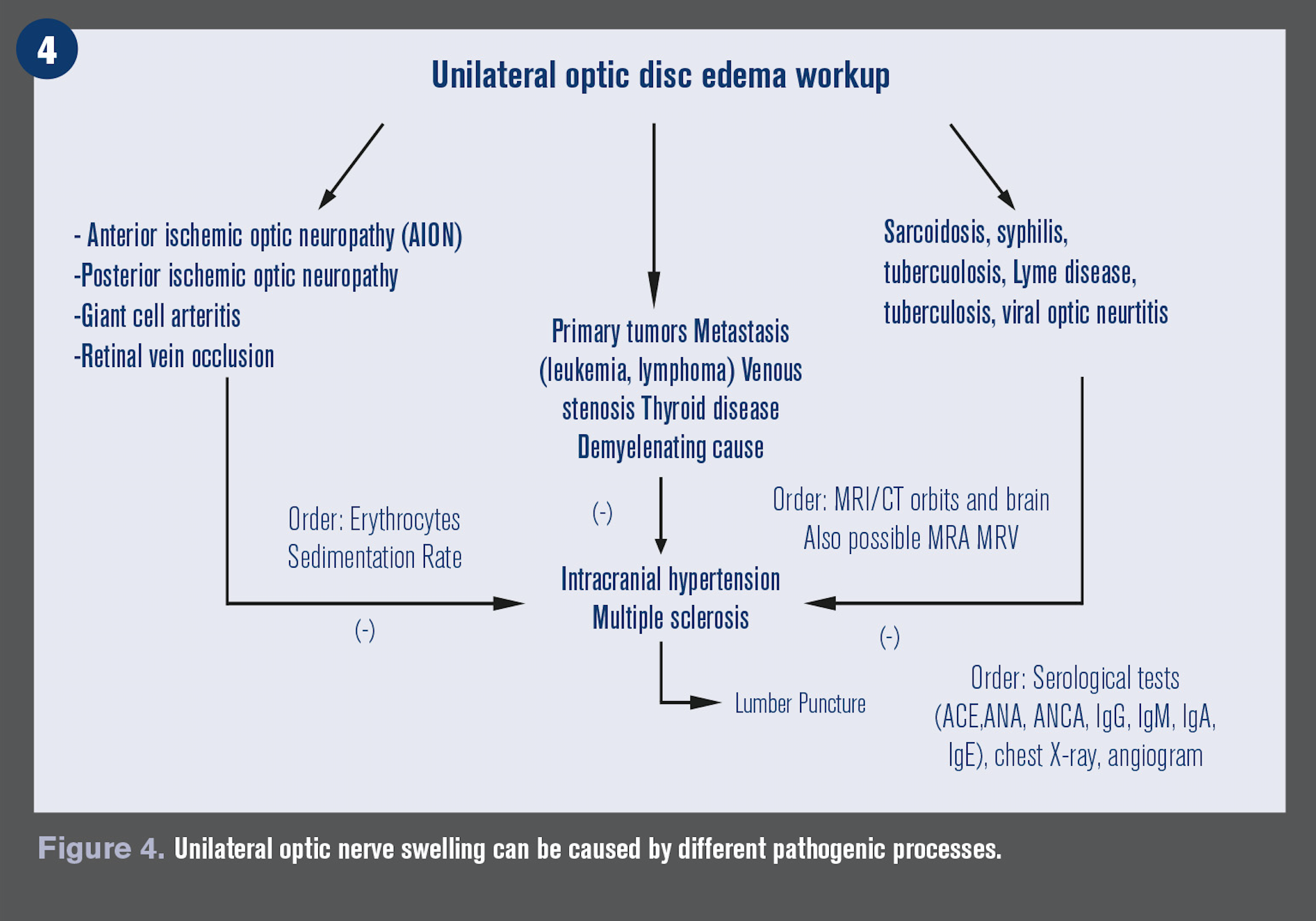
Unilateral optic nerve swelling
Unilateral optic nerve swelling can be caused by different pathogenic processes, including demyelinating, vascular, compressive, inflammatory, infectious, infiltrative, toxic, nutritional, and hereditary causes. (see Figure 5)
Demyelinating or optic neuritis (ON): History of sudden vision loss in one eye and pain associated with eye movement, prior history of neurological symptoms such as paresthesia, limb weakness, ataxia, chronic arm paresthesia, and diminution of vision following a rise in body temperature such as after exercise or a hot shower (Uhthoff’s phenomenon) is very common.
ON improves in 90 percent of cases over several weeks to near normal acuity. A brain MRI is needed to identify the presence of white matter lesions to confirm diagnosis.15
Arteritic ischemic optic neuropathy (AION): The presence of preceding transient visual loss, diplopia, temporal pain, jaw claudication, fatigue, weight loss, and myalgias is strongly suggestive of AION due to giant cell arteritis (GCA).
GCA causes only 6 percent of ischemic optic neuropathy, but it is visually devastating-a quick diagnosis can initiate treatment and prevent vison loss in the lateral eye. Other causes include polyarteritis nodosa, Wegener’s granulomatosis, and connective tissues disorders.15
Non-arteritic AION is usually less severe and the most common cause of unilateral optic nerve edema in those >50 years. Usually associated with poor blood circulation to the optic nerve and associated with diabetes mellitus, glaucoma, hypercholesterolemia, and history of drug use.16
Compressive: Lesions of the orbit and, less commonly, the optic canal can lead to optic nerve damage, and the visual loss is usually gradual and progressive. Common causes include optic glioma, meningioma, lympangiomas, pituitary adenomas, craniophyrangiomas, and Grave’s orbitopathy. MRI and CT of brain and orbit are crucial for diagnosis.17,18
Inflammatory: A variety of systemic auto-immune disorders can cause optic nerve swelling. These include sarcoidosis, Bechet’s disease, systemic lupus erythematosus, Sjögren’s syndrome, Wegener’s granulomatosis, and syphilis. Laboratory for each diagnosis should be included in the workup on these patients.19,20
Infiltrative: The optic nerve can be infiltrated by secondary tumors and malignancies, including metastasis, carcinomas, leukemia, lymphoma, and multiple myeloma. Patients with a history of cancer and acquired optic nerve edema should be considered cancerous until proven otherwise. Neuroimaging should be ordered to help determine the correct diagnosis.21,22
Infectious: Bacterial, viral, and fungal infections can lead to optic nerve disease and edema. The most common causes are toxoplasmosis, Bartonella (Cat-scratch disease) and Lyme disease. Laboratory testing and good case history is important in isolating the causative pathogen.23,24
Nutritious/toxic: Various drugs, toxins, and nutritional deficiencies can lead to optic nerve disease. These typically mimic and cause a secondary IIH. These include tetracyclines, Vitamin A, amiodarone, and lithium.
Hereditary: The hereditary optic neuropathy that causes discs to appear swollen is Leber’s hereditary optic neuropathy and typically occurs between ages of 15 and 35.27 Genetic testing and counseling should be considered if this is the suspected cause of optic neuropathy.
References:
1. Scott C, Kardon R, Lee A, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010 Jun;128(6):705-11.
2. Behbehani R. Clinical approach to optic neuropathies. Clin Ophthalmol. 2007 Sep;1(3):233-46.
3. Friedman DI. Papilledema. In: Miller NR, Newman NJ. Walsh and Hoyt’s Clinical Neuro-Ophthalmolgy, 6th Ed. Baltimore: Lippincott Williams and Wilkins, 2005:237-291.
4. Friedman D, Jacobson D. Idiopathic intracranial hypertension. J Neuroophthalmol. 2004 Jun;24(2):138-45.
5. Avery R. Interpretation of lumbar puncture opening pressure measurements in children. J Neuroophthalmol. 2014 Sep;34(3):284-7.
6. Shah A, Szirth B, Sheng I, Xia T, Khouri AS. Optic disc drusen in a child: diagnosis using noninvasive imaging tools. Optom Vis Sci. 2013 Oct;90(10):e269-73.
7. Pane A, Miller N, Burdon M et al. The neuro-ophthalmology survival guide. Mosby; 2007.
8. Carta A, Bertuzzi F, Cologno D, Giorgi C, Montanari E, Tedesco S. Idiopathic intracranial hypertension (pseudotumor cerebri): Descriptive epidemiology, clinical features, and visual outcome in parma, italy, 1990 to 1999. Eur J Ophthalmol. 2004 Jan-Feb;14(1):48-54.
9. Keltner JL, Johnson CA, Cello KE, Wall M; NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Invest Ophthalmol Vis Sci. 2014 Apr 29;55(5):3200-7.
10. OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group, Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, Keltner J, Kupersmith MJ, Sibony P, Plumb K, Wang JK, Werner JS. OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014 Nov 4;55(12):8173-9.
11. Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology. 2011 Feb;118(2):412-4.
12. Auw-Haedrich C, Staubach F, Witschel H. Optic Disk Drusen. Surv Ophthalmol. 2002 Nov-Dec;47(6):515-32.
13. Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography.
Arch Ophthalmol. 2009 Jan;127(1):45-9
14. Benjamin I, Alexander LJ. Congenital and acquired anomalies of the optic nerve head. In: Primary Care of the Posterior Segment. 3rd ed New York: McGraw-Hill. 2002:209-315.
15. Beck RW. The optic neuritis treatment trial: three-year follow-up results. Arch Ophthalmol. 1995 Feb;113(2):136-7.
16. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998 Apr;125(4):509-20.
17. Miller NR, Newman NJ, Biousse V, et al (eds). Walsh and Hoyt’s Clinical Neuro-Ophthalmology: The Essentials. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
18. Lee AG. Neuroophthalmological management of optic pathway gliomas.
Neurosurg Focus. 2007;23(5):E1.
19. Jabs DA, Miller NR, Newman SA, Johnson MA, Stevens MB. Optic neuropathy in systemic lupus erythematosus. Arch Ophthalmol. 1986 Apr;104(4):564-8.
20. Kansu T, Kirkali P, Kansu E, Zileli T. Optic neuropathy in Behçet's disease. J Clin Neuroophthalmol. 1989 Dec;9(4):277-80.
21. Brown GC, Shields JA, Augsburger JJ, Serota FT, Koch P. Leukemic optic neuropathy.
Int Ophthalmol. 1981 Mar;3(2):111-6.
22. van Oostenbrugge RJ, Twijnstra A. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology. 1999 Jul 22;53(2):382-5.
23. Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (Lond). 2007 Jun;21(6):746-51.
24. Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, Umeda A, Furuya T, Kawauchi S, Sasaki K. Cat scratch disease: Analysis of 130 seropositive cases. J Infect Chemother. 2002 Dec;8(4):349-52.
25. DeVita EG, Miao M, Sadun AA. Optic neuropathy in ethambutol-treated renal tuberculosis. J Clin Neuroophthalmol. 1987 Jun;7(2):77-86.
26. Rizzo JF, 3rd, Lessell S. Tobacco amblyopia. Am J Ophthalmol. 1993 Jul 15;116(1):84-7.
27. Votruba M, Thiselton D, Bhattacharya SS. Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. Br J Ophthalmol. 2003 Jan;87(1):48-53.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.