Incorporating meibomian gland imaging
Adding this technology can improve your diagnosis and better educate your patients
Dry eye disease (DED) is nothing new, but how eyecare practitioners diagnose and monitor DED has evolved due to new technology. In-office laboratory tests such as TearLab Osmolarity System and RPS InflammaDry and equipment such as Oculus Keratograph 5M or TearScience LipiView give us better information to manage our dry eye patients.
Optometry and dry eye
Establishing a dry eye clinic in your practice does not require a large financial investment, nor does it require a marketing campaign for potential dry eye patients. Nearly half of all U.S. adults (about 48 percent) experience one or more dry eye symptoms regularly, according to an Allergan survey,1 so many of your current patients will benefit from your ability to properly diagnose and treat their DED.
Optometrists are perfectly positioned to be the primary eyecare provider for those millions of dry eye sufferers because a common complaint for these patients is blurry vision.
Today’s digital age provides many benefits such as computers, smartphones and tablets as well as the disadvantage of increasing dry eye symptoms caused by the reduced blink rate associated with up-close tasks.
If you ask your patients the right questions, you will elicit the complaints caused by tear film deficiency and meibomian gland dysfunction (MGD). Performing the right tests enables you to diagnose and treat the ocular surface disease, providing comfortable and clear vision for your patients suffering from DED.2
Identifying MGD
The Tear Film & Ocular Surface Society (TFOS) International Workshop on Meibomian Gland Dysfunction formally defined MGD as a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion.2
It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease. An optimum level of meibomian gland expression is required for tear stability.
Patients can present with various degrees of MGD and aqueous deficiency-of the two currently accepted forms of dry eye, evaporative dry eye is thought to be significantly more common than aqueous-deficient dry eye. It appears that abnormalities in meibomian gland structure or tear film quality/quantity are the leading contributors to dry eye disease.
For this reason, evaluation of the meibomian glands has become a major factor in determining the cause and severity of tear film insufficiency in dry eye patients.
In addition to measuring the non-invasive tear break-up time (TBUT), tear osmolarity, and ocular surface inflammation, a good clinician must also grade the quality of the meibomian gland structure and quantity of healthy glands. Grading meibomian gland dysfunction is primarily based on the amount of meibomian gland dropout.3
A grading scale was established in 2012 by Dr. Heiko Pult. It is based on a scale of 0-4 with Degree 0 being no gland loss, Degree 1 <25 percent gland loss, Degree 2 26 to 50 percent gland loss, Degree 3 51 to 75 percent loss, and Degree 4 >75 percent gland loss.4
Meibography in practice
Prior to implementing meibography as a part of my dry eye workup, I would rely on the slit-lamp biomicroscopic examination of the eyelids for assessing the meibomian gland structure and function. I would perform a non-invasive examination of the eyelid for evidence of irregular lid margins as evidenced by hyperemia, telangiectasias, vascular engorgement, meibum orifice plugging, and digital expression of meibum from meibomian glands by applying gentle pressure with my finger, cotton swab, or expressor paddle.
These changes in eyelid appearance and meibum expression correlate with dysfunction of the meibomian glands. While this method is frequently used in the clinical setting as part of the standard slit-lamp examination of the eyelid, it is limited as an indirect measure of meibomian gland structure and function.5-7
Earlier attempts to perform meibography involved use of a handheld transilluminator that often proved to be uncomfortable for the patient.8
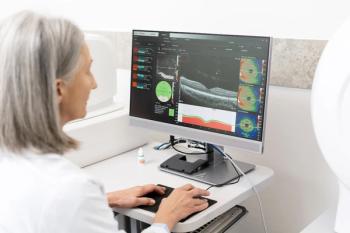
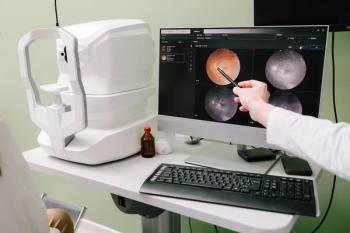
Today’s meibography incorporates an infrared LED light to capture an image of the meibomian glands of the inferior and superior lids. It allows a detailed picture of meibomian gland structure, including those glands that have increased tortuosity or have stopped functioning, resulting in meibomian gland dropout (See Figure 1).
Imaging for patient education
Instruments like Keratograph 5M or LipiView enable us to perform non-invasive meibography with little to no patient discomfort. I primarily utilize Keratograph 5M for meibography to assess meibomian gland structure and to educate my patients on their ocular condition by showing them an in vivo image of their meibomian glands.
Meibography can be repeated multiple times on the same patient over a period of time, enabling the clinician to document progression of MGD. If I can demonstrate to the patient that my prescribed treatment plan is successful, it may motivate the patient to be more compliant with therapy, resulting in a better outcome.
If I can show the patient his meibomian gland dropout has stabilized over the course of his therapy, I can develop a positive doctor/patient relationship that will build a loyal patient following for my practice.
Patient education is essential for patient compliance on their prescribed therapy. These images provide an easy way to show patients what is normal vs. abnormal and allow me to explain in detail the cause of their dry eye symptoms.
A patient’s commitment to follow through with treatment directions is critical for successful treatment of chronic diseases such as DED and MGD, and I use every means available to encourage them to follow my prescribed course of treatment.
I explain to my patients that their treatment will be ongoing over a period of months or longer, comparable to a long-distance race instead of a short sprint. It is critical to set appropriate expectations for the treatment outcome because MGD is a chronic condition and may require a multi-step treatment plan to control or stabilize the condition.
Often, complete remission of MGD is not possible, resulting in ongoing treatment over the latter years for many geriatric patients.
Meibography in your dry eye protocol
As part of your dry eye clinic you must develop a protocol that allows you to identify, diagnose, and treat MGD. Meibography can be an important part of that protocol.
I perform meibography to evaluate the quality and quantity of the meibomian glands of the inferior and superior lids. The test is easily performed by a technician during the patient pretest and may be reviewed by the doctor in the exam room with the patient.
I perform the test on contact lens wearers who experience dry eye symptoms. This allows me to slow down contact lens dropout because I can diagnose and treat the underlying MGD before it interferes with a contact lens wearer’s ability to comfortably wear his lenses.
Before referring cataract patients for surgical intervention, it is necessary to treat any DED that may potentially interfere with a positive surgical outcome. Monitoring progression of MGD requires the patient to return at a future date to repeat meibography.
Comparing meibography tests on the same patient over time shows whether the MGD is stable or progressing and provides direction for a therapy regimen. Once a diagnosis of MGD is established, a treatment plan may include a Bruder mask, lipid-based artificial tears, oral doxycycline, and meibomian gland expression.
Meibography is a billable bilateral procedure for most medical insurance plans using the CPT code 92285 (external ocular photography). Numerous anterior segment ICD-10 codes may be used for reimbursement for 92285.
A quick review of your insurance carriers for this procedure will provide you a list of ICD-10 diagnosis codes to use. Average Medicare allowable for 92285 is $21.43.
Meibomian gland dysfunction resulting in DED is garnering more attention from optometrists due to the larger number of sufferers caused by the increased use of digital devices by millions of people from young to old.
Meibography is now an established procedure for identifying, diagnosing, and monitoring the many patients with MGD.
References:
1. American Optometric Association. New Allergan survey shows 48% have dry eye symptoms. AOA News. 2011;50(4):37.
2. Miljanoviæ B, Dana R, Sullivan DA, et al. Impact of dry-eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007 Mar;143(3):409-15.
3. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1922-9.
4. Bron A.J, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond). 1991;5 ( Pt 4):395-411.
5. Hykin PG, Bron, AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992 Jul;11(4):334-42.
6. Norn, M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh). 1987 Apr;65(2):137-42.
7. Den S, Shimizu K, Ikeda T, et al. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006 Jul;25(6):651-5.
8. Hom M. How to perform in-office meibography. Rev Optom. 2002 Jul 15. Available at: https://www.reviewofoptometry.com/article/how-to-perform-in-office-meibography. Accessed 9/8/16.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.