A postoperative problem solver: Presbyopia-correcting drops
There will be very little downside to optometrists and their practice in offering presbyopia-correcting drops to patients who want better vision without glasses or contact lenses.


Presbyopia, the age-related loss of near vision, has long vexed patients and eye doctors. However, the first of a raft of new presbyopia-correcting eyedrops recently hit the market, with a number of others slated to go up for FDA approval in the next few years.
Presbyopia-correcting drops represent a whole new product category—one with a lot of upside for optometrists who want to help their patients see well at all distances.
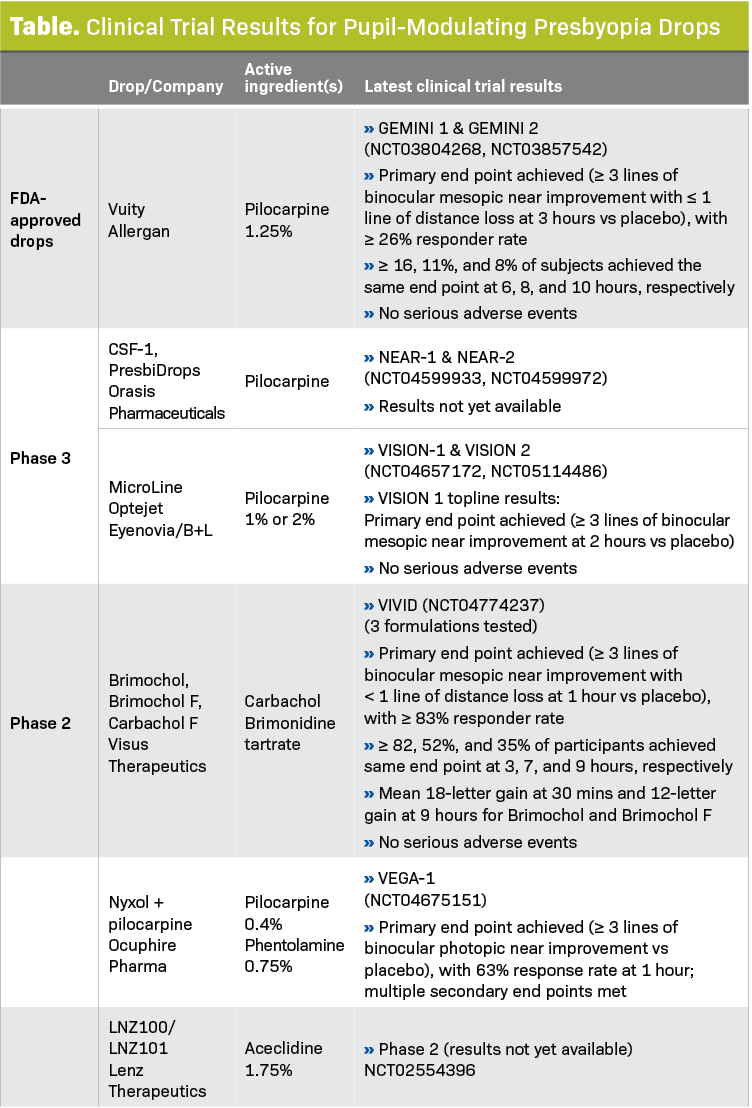
The first drop introduced was Vuity (Allergan, an AbbVie company). Additional offerings from Visus Therapeutics, Orasis Pharmaceuticals, Ocuphire Pharma, Lenz Therapeutics, and Eyenovia are still under investigation (Table).
Not only do these miotic, pupil-modulating drops have the potential to bring many young patients with presbyopia into our practices, they can also help us address postoperative problems that have been tricky to solve. For example, we know that the vast majority of patients undergoing cataract surgery don’t take advantage of presbyopia-correcting IOLs, due to cost or lack of eligibility. To me, helping these patients with standard monofocal IOLs achieve seamless vision is one of the greatest opportunities we have.
Patients who see well at a distance already may not need glasses much at all, but presbyopia-correcting drops could help those with pseudophakia regardless of their refractive posture. A distance-corrected individual could use the drops to avoid the fall risks associated with bifocals and progressive spectacles, for example.
Additionally, we all have those disappointed patients in our practices who paid out of pocket for premium IOLs…and were underwhelmed by the results. Maybe they had an early bifocal lens that didn’t provide intermediate vision or extended depth-of-focus (EDOF) IOLs that provide great quality “walking around” vision—regardless, they could benefit from better reading vision. By causing a smaller pupil, miotic drops could even eliminate some of the dysphotopsia symptoms that plague a small percentage of patients implanted with diffractive IOLs.
I believe that presbyopia-correcting drops are going to nicely fill the gap for all dissatisfied patients with pseudophakia.
Making the most of monovision
For patients who have surgical monovision, whether from prior LASIK or cataract surgery, we could consider using presbyopia-correcting drops in just the distance (dominant) eye or in both eyes, to provide better intermediate and near vision.
An exciting feature of miotics, as topical presbyopic correction, is that they likely won’t compromise distance vision, which makes them an option for those who just have 1 eye corrected for distance.
So far, data suggest that new formulations of drugs such as pilocarpine and carbachol are able to reduce the pupil size by about half, with a near vision gain of 2 to 4 lines and less than 1 line of distance vision loss. I am confident that most of our patients are going to be able to function really well with these drops at distance, even in mesopic lighting conditions.
Some patients can’t adapt to the disparity between the 2 eyes with monovision. Others struggle with glare and halo at night because of the disparity. Before I would recommend giving up on monovision and surgically correcting it on the cornea (which has its own risks and disadvantages), I would prefer to try pupil-modulating drops to see if they relieve the patient’s problems.
Historically, one of our treatment options for patients struggling with glare and halo has been to use topical brimonidine, a sympatholytic alpha-2 agonist, to stabilize the pupil and reduce glare. It is entirely possible that presbyopia-correcting drops with similar effects could help reduce night vision symptoms. Because these drops act on the iris, their presbyopic effect is much closer to the eye’s nodal point than a corneal correction, which may also end up resulting in a “truer” version of monovision for our patients who would otherwise seek an enhancement.
Opening up the presbyopia conversation
Discussion around presbyopia has always been one of the most difficult conversations to have with patients. Losing the ability to read menus or see phones is one of the first harbingers of old age—and it’s not a welcome one. Because that conversation can be so fraught and, frankly, confusing for patients to understand, many of us avoid “the presbyopia talk” until absolutely necessary.
With the proliferation of more options for presbyopia correction at every stage, including noninvasive pupil-modulating drops, I would love to see our profession proactively discuss presbyopia much earlier in the game and truly embrace the notion of seamless vision for life.
I think patients are going to be excited when they hear about these drops, and they should hear about them from us. Optometrists should be at the forefront of educating our communities about presbyopia and advances in treatment.
Does that mean our patients will abandon their current modalities of presbyopic treatment? No, I don’t think so. I regard these topical drops not so much as cannibalizing the practice’s presbyopia business as catapulting it to the next level. Patients want choices and options, and the reality is that 50% of presbyopic patients never even get an eye exam.1,2
The availability of prescription drops for near vision will elevate the importance of seeing an eye doctor. And when the patient is in your office, they will realize there are other options that are also better than drug store “cheaters.” I firmly believe that attracting more patients with presbyopia into the office will ultimately pay off regarding retail sales, optometric exam billing, and happier patients.
Customized care
Being able to provide a therapy that allows patients to pick and choose when they want to use it is very satisfying. Pupil-modulating drops don’t take weeks or months to reach peak efficacy; rather, they work in minutes. There won’t be any health consequences to skipping doses. Furthermore, the patients will have the same results weeks to months after starting the medications.
In the GEMINI clinical trials of the first available presbyopia-correcting drop, Vuity, the drop met its end point of improved near vision at 3 hours (26%-31% of patients met this end point, statistically significantly more than in the control group).3
Although 16% to 18% of patients still had good near vision at 6 hours, the difference wasn’t statistically significant. There were no serious adverse events. Headache and eye redness, occurring in more than 5% of patients, were the most common nonserious events.3
Future presbyopia-correcting drops may have an even longer duration of effect. An early readout of Phase 2 data from the VIVID clinical trials for Brimochol (Visus Therapeutics), for example, showed that 82%, 52% and 35% of patients maintained a 3-line gain in near vision at 3, 7, and 9 hours, respectively.4
We will likely reach a point at which patients can instill drops in the morning and have good vision for their entire workday. Some may want to use the drops only during the week at work, whereas others don’t mind glasses when sitting at a desk but would like to be unencumbered for exercise or going out on weekends. This solution doesn’t have to be one size fits all.
When we consider putting patients on any new medication, we need to find the best population of patients with the attributes to be successful. Furthermore, we always have to weigh the risks and benefits.
In this case, I expect the benefits will be high and the risks extremely low. There will be very little downside to optometrists and their practice in offering presbyopia-correcting drops to patients who want better vision without glasses or contact lenses.
References
1. 2018 American Eye-Q Survey. Edelman Intelligence. January 2019. Accessed March 18, 2022. https://higherlogicdownload.s3.amazonaws.com/MBGH/4f7f512a-e946-4060-9575-b27c65545cb8/UploadedImages/Eye_Health_TK/2018_American_Eye-Q_Results_1_23_19.pdf
2. Vision Service Plan. Surprising Eye Health Stats. Accessed March 18, 2022. https://www.vsp.com/eyewear-wellness/eye-health/eye-health-survey-results
3. Abbvie RXABBVIE. Vuity Prescribing Information. Accessed March 18, 2022. https://www.rxabbvie.com/pdf/vuity_pi.pdf
4. Visus Therapeutics. Visus Therapeutics announces positive topline clinical data from phase 2 VIVID study of BRIMOCHOL for the treatment of presbyopia. November 30, 2021. Accessed March 18, 2022. https://uploads-ssl.webflow.com/61088b48cbb6c4347abaaca5/61a59e76db9866f53d6a005b_Visus%20Therapeutics%20Brimochol%20Phase%202%20Results_11292021%20FINAL.pdf
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.




.png&w=3840&q=75)











