AGN-190584 is a novel treatment for patients with presbyopia
New eye drop is first designed to improve near vision
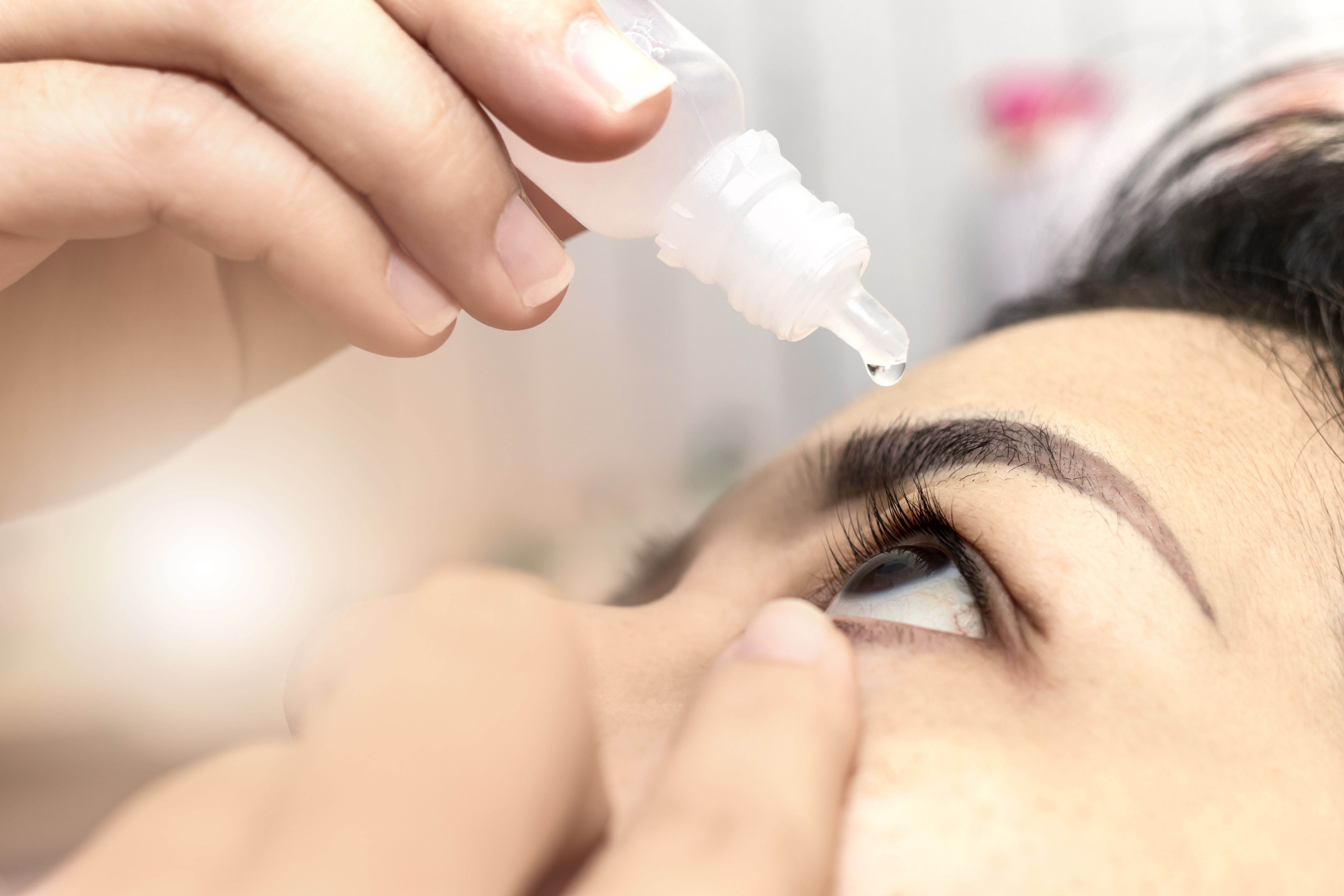
Data from the GEMINI 1 clinical study evaluating Allergan’s AGN-190584 for the treatment of presbyopia were presented at the American Society for Cataract and Refractive Surgery annual meeting. The new data suggests AGN-190584 could be a novel treatment for the most common and progressive form of vision loss and demonstrated that AGN-190584 met its primary and secondary efficacy endpoints.
How it works
AGN-190584 is a non-surgical treatment option that can treat presbyopia. According to the company, AGN-190584 is a potential first-of-its-kind treatment, which can affect the ability of patients with presbyopia to focus on near objects. Results from a phase 3 study showed thatAGN-190584 provided improved near vision without affecting distance vision.
“If approved by the FDA, AGN-190584 is expected to be the first eye drop specifically designed for presbyopia," said Michael Robinson, MD, vice president, global therapeutic area head, eye care, AbbVie, in a statement. "We are pleased with the favorable safety and efficacy results, as well as the rapid onset and duration of improvement in near and intermediate vision without impacting distance vision, from the Phase 3 GEMINI 1 clinical study."1
The data from the GEMINI 1 study, which was combined with those from the GEMINI 2 trial, formed the basis for the company's new drug application.
Results of the AGN-190594 trial showed that patients who were treated with AGN-190594 reported significant improvement in near-vision reading and satisfaction.
Reference
1. New data presented on the safety and efficacy of investigational AGN-190584 as a potential novel treatment for presbyopia, a common and progressive eye condition. Biospace. July 25, 2021. Accessed July 27, 2021. https://www.biospace.com/article/releases/new-data-presented-on-the-safety-and-efficacy-of-investigational-agn-190584-as-a-potential-novel-treatment-for-presbyopia-a-common-and-progressive-eye-condition/