Aramis Biosciences doses first patient in phase 2 trial for DED treatment
Topline proof-of-concept data are expected by the first quarter of 2023.
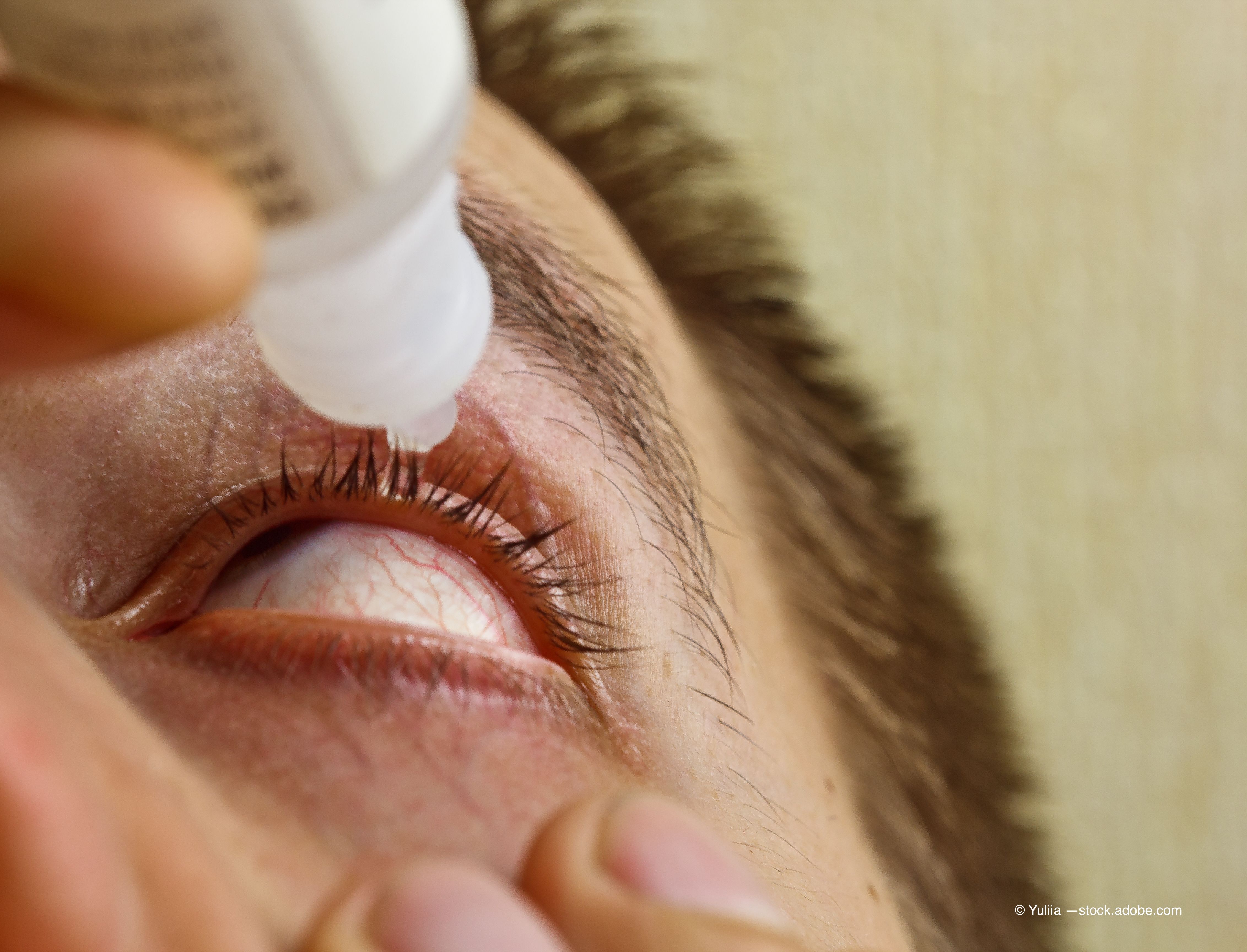
Aramis Biosciences announced Monday that the first patient has been dosed in its phase 2 trial analyzing A197, a novel first-in-class, topical immunomodulatory agent, for the treatment of dry eye disease (DED).
The phase 2, multi-center, double-masked, randomized, vehicle-controlled clinical trial (NCT05238597) is evaluating the safety and tolerability of A197.
Two doses of A197 ophthalmic solution will be distributed in approximately 200 patients diagnosed with DED, according to a company news release.
Primary endpoints are measuring the change from baseline in corneal fluorescein staining. A key secondary endpoint is evaluating eye dryness symptoms using the visual analog scale.
An estimated 20 centers across the United States will participate in the trial.
Topline proof-of-concept data is expected by the first quarter of 2023, according to David S. Tierney, MD, Aramis Biosciences’ president and CEO.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.