Cloudbreak Pharma survey shows patient frustration in treatment options for pterygium
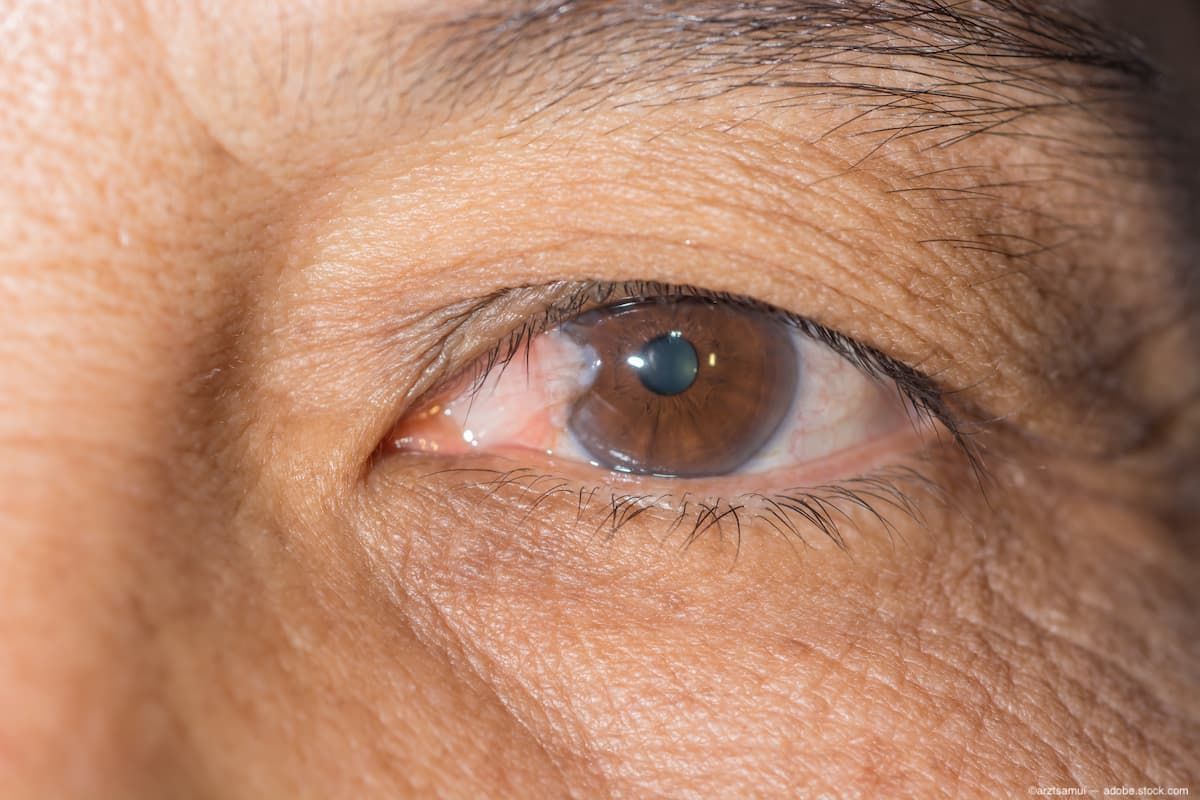
Pterygium is an ocular disease commonly referred to as “surfer’s eye,” which can result from exposure to high levels of UV radiation.
Image Credit: AdobeStock/arztsamui
A recent survey by Cloudbreak Pharma conducted on patients with pterygium highlighted the patient burden and showed a need for non-surgical treatment options.1
Pterygium is an ocular disease commonly referred to as “surfer’s eye,” which can result from exposure to high levels of UV radiation. It is a fibro-vascular growth that extends from the conjunctiva to the corneal surface and affects roughly 15 million people in the US. Only 1 in 6 patients suffering from the disease have been diagnosed, according to the company.1
Currently, there is no pharmacological treatment approved for pterygium. In turn, according to the company, patients may turn to over-the-counter topical treatments to provide symptomatic relief or surgery which could potentially damage the eye, and may not correct vision, redness, and irritation.1
“Pterygium is far more common and has far more impact on patients – than implied by the casual name, ‘surfer’s eye.’ Chronic symptoms can impose a daily burden on people grappling with the disease, and unfortunately, many people who experience symptoms face challenges receiving a formal diagnosis,” said Abu Abraham, CMO of Cloudbreak Pharma. “Cloudbreak Pharma recognizes the urgent and growing need for new non-surgical treatment options to address the debilitating symptoms associated with pterygium and help delay or avoid the need for surgery for these patients for whom there have been so few options.”
The survey was conducted in volunteers for a multi-center Phase 3 clinical trial enrolling people with a known pterygium of ≥1.2 mm and conjunctival hyperemia (measured on a scale of 0-4) in the US.
Key takeaways from the survey:
- Many respondents reported low satisfaction with available treatment options and said relief was only temporary.
- Nearly 80% of patients experienced challenges learning about pterygium and building relationships with their care providers.
- Most were be diagnosed quickly when seeking care, although 50% of survey respondents did not seek care for 2+ years from symptom onset.
A Phase 2 clinical trial of Cloudbreak Pharma’s CBT-001 (NCT03049852) was completed in 2020 and showed promising results, including its potential to reduce lesion length and decrease pterygia vascularity for patients with pterygium. CBT-001 is currently being investigated in Phase 3 clinical studies to determine its ability to slow or stop disease progression and potentially eliminate or postpone the need for eye surgery for patients with pterygium.1
Reference:
New Survey Results from Cloudbreak Pharma Highlight Disease Burden, Need for Non-surgical Treatment Options for Underdiagnosed and Undertreated Ocular Disease. Press release; April 5, 2024. Accessed April 9, 2024. https://www.businesswire.com/news/home/20240403467915/en/New-Survey-Results-from-Cloudbreak-Pharma-Highlight-Disease-Burden-Need-for-Non-surgical-Treatment-Options-for-Underdiagnosed-and-Undertreated-Ocular-Disease