Determining the value in genetic testing for AMD
For any treatment to be successful, early detection is key. For that reason, genetic testing may play a role in determining the frequency of follow-up in patients who are determined to have high-risk alleles.

Note this clinical scenario: A 70-year-old man presents for an eye exam. He is noted to have a few small macular drusen and best-corrected vision of 20/20 in each eye. He is followed annually by the same doctor for 20 years with a take-home Amsler grid. The only changes noted throughout the years are a slight increase in the number of drusen with no decrease in visual acuity; Amsler grid testing is always negative. A few months after his last annual exam, at age 90, he notices at home that the vision in his right eye is blurry” when watching TV, but he thinks he is developing a cataract like his friends. He thinks he will wait until his next annual exam; he has misplaced his Amsler grid; he hates being dilated.
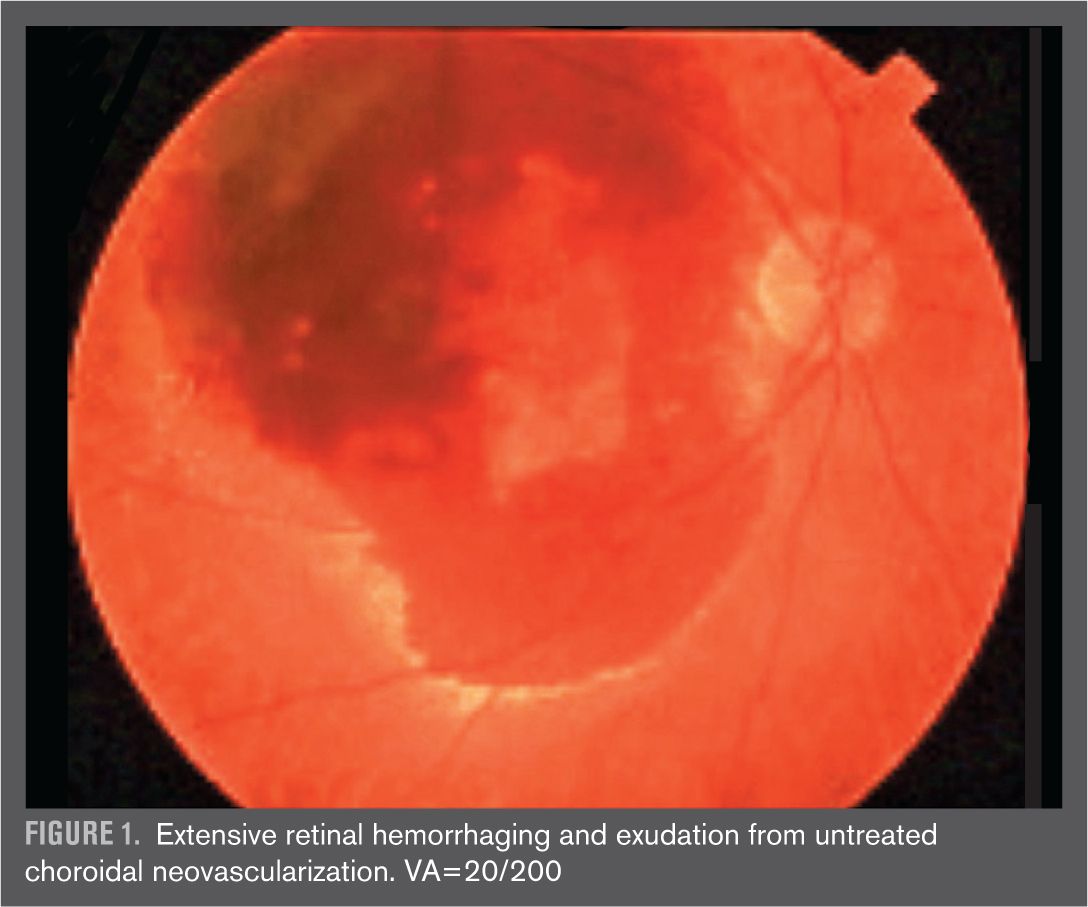
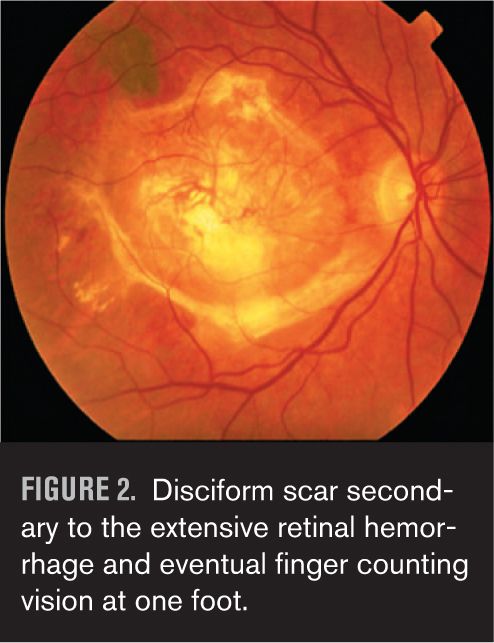
Three months later, the vision decreases markedly and even though it wasn’t time for his annual exam, he thinks he ought to see his eye doctor because the “cataract” must have gotten a lot worse. To his surprise and dismay, his eye doctor tells him he has developed the wet form of macular degeneration and the amount of bleeding in the back of his eye is so great at this point that nothing can be done (Figure 1). He eventually develops a scar and finger-counting vision (Figure 2). This was especially frustrating because the patient was the eye doctor’s own father. Remarkably, he lives to over 100 years, complaining for the last decade of his life about the dark blind spot right in the center of his vision.
Current views on genetic testing for AMD
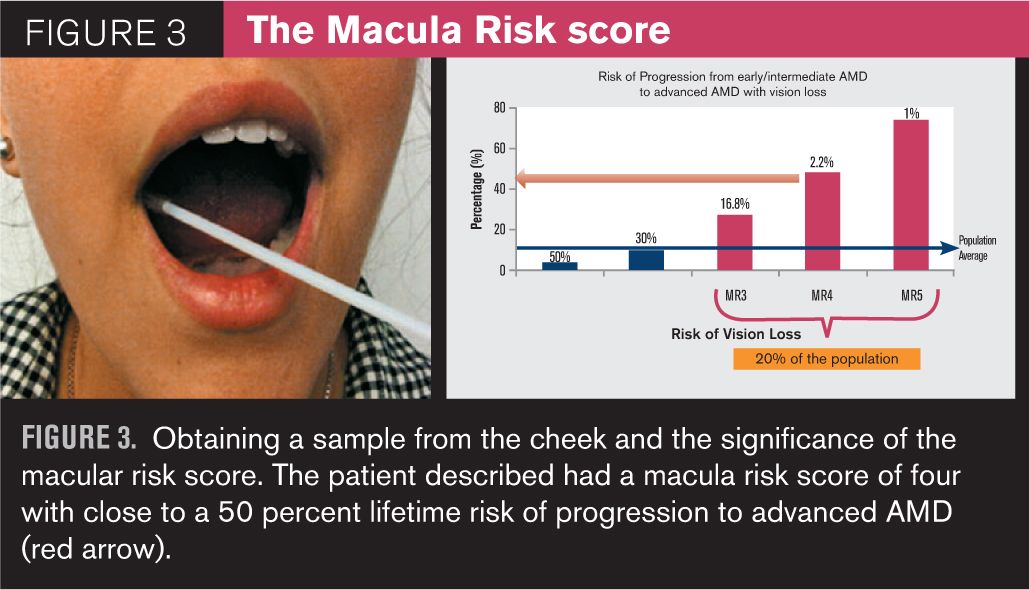
Years later, this eye doctor learns of a simple genetic test for patients with signs of age-related macular degeneration (AMD) that involves taking a sample from the inside of a patient’s cheek using a brush. The sample is analyzed within a few weeks to determine if a patient has certain high-risk alleles that will increase the risk of lifetime progression to a more advanced form of AMD and thereby, along with smoking history, is used to determine a risk category and frequency of follow-up.
The risk categories range from one to five. A risk category score of one or two means that the patient is at no greater risk than the general population to progress to a more advanced form of AMD and could be followed every six months to a year. A risk category score of three, four, or five means that the patient is at a higher risk than the general population to progress and should be followed more frequently, perhaps every three to four months. The eye doctor decides to test this now 90-year-old patient who was followed for the past 20 years. The results indicate a risk category score of four, which meant that this eye doctor would have followed this patient every three to four months (had this information been available at the time) and not just once a year. It is likely that the patient’s wet AMD would have been detected much earlier, and his vision might have been easier to save because by the time this patient developed choroidal neovascularization (CNV), effective clinical intervention was already available.
AMD is the leading cause of irreversible blindness in people over 55 in the developed world. Of the estimated 9.1 million people who have AMD in the U.S., approximately 1.75 million people have late-stage disease and another seven million are at high risk of developing advanced disease.1 About 90 percent of patients with AMD exhibit the dry, atrophic form of the disease, for which there is currently no clinical intervention, with the exception of the Age-Related Eye Disease Study (AREDS) formulation which reportedly reduces risk to advanced AMD by 25 percent in patients with intermediate AMD. About 10 percent of affected patients with AMD develop CNV, which is responsible for 90 percent of severe vision loss. The potential for vision loss from all forms of AMD increases if the disease is undetected, untreated, unsuccessfully treated, or inappropriately treated.2
Genetic testing allows for early detection
For any treatment to be successful, early detection is key. For that reason, genetic testing may play a role in determining the frequency of follow-up in patients who are determined to have high-risk alleles, as exemplified in the scenario above. Patients like the one above with no risk factors and with just a few small macular drusen and good visual acuity are typically followed once a year. But one phenotype (a few small drusen) with differing genotypes may require different follow-up regimens.
The progression of AMD is difficult to predict in any given patient because there are a number of factors that contribute to the risk of development and progression of this disease. Some risk factors can be changed, such as diet, smoking habits, body mass index, and cholesterol levels. Other factors are fixed, such as age, gender, and family history (genotype).
In 2005, three landmark studies were published that confirmed an association of certain variations in specific genes that increased the relative risk of the development and progression of AMD. Specifically, variations in the complement factor genes (CFH and C3), ARMS2 genes, and other mitochondrial genes have been identified that play a role in the regulation of inflammation in the retina.3-5 So far, more than 20 genetic variants that influence AMD risk have been identified.
In the last few years, noninvasive genetic testing for the presence of the more common mutations or single nucleotide polymorphisms (SNPs) associated with increased risk of AMD progression has become a reality. Obtaining samples from the patient does not involve blood draw, which is not available to most OD practices. Instead, the test involves obtaining a cheek swab using two brushes, one for each side of the mouth, and the kit comes with a pre-addressed envelope. The results, which include a macula risk score of progression to advanced AMD, are available within a few weeks. The higher the score, the greater is the risk. Many practitioners have already been using these tests in their patients with drusen and/or diagnosed AMD to help determine frequency of follow up, but more recently, genome- directed therapy, or GDP, has been reported to determine the optimal nutritional supplementation for the patient with intermediate AMD.6
Commercial availability
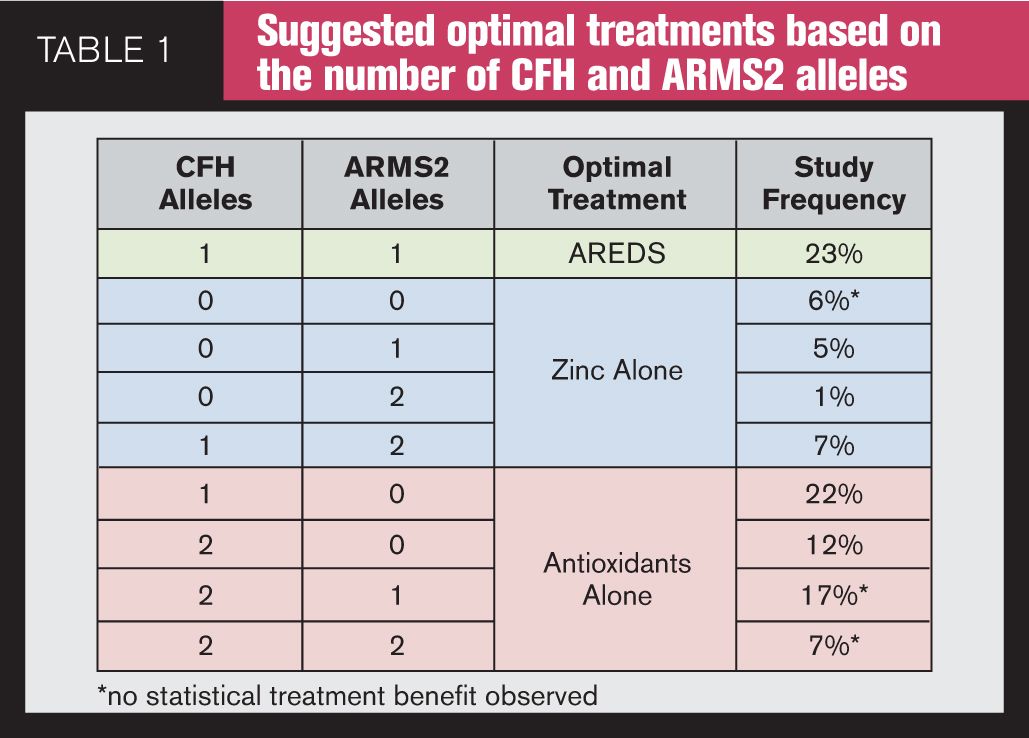
Genetic testing for AMD involves taking a sample from the inside of the patient’s cheek during an office visit and sending it to a commercial genetic testing laboratory. If the patient has drusen and/or AMD, in the U.S., insurance will cover the cost of the testing. Analysis is performed, and a report is available in about three weeks. Currently, two companies offer commercial genetic testing for AMD: Macula Risk NXG test from ArcticDx and RetnaGene from Nicox. These tests predict a patient’s risk for progression to advanced AMD within two, five, and 10 years using an analysis of genetic variants (SNPs) associated with AMD, clinical AMD status, and significant non-genetic risk factors, smoking, and for Macula Risk NXG, body mass index (BMI). In 2013, ArcticDx added its Vita Risk pharmacogenetic analysis to the Macula Risk NXG test; it provides a genotype-directed selection of appropriate ocular vitamin treatments for intermediate AMD patients based on their testing results (Table 1). Vita Risk is also available as a stand-alone test.
Genetic testing can guide personalized AMD treatment
The value of genetic testing
The question eyecare clinicians have been asking for years is why some patients progress to CNV and geographic atrophy (GA), and how can we predict which patients will progress? In addition, why do some patients respond very well to treatment of CNV with anti-VEGF, while others do not? The answer most certainly involves a list of factors, including phenotypic risk factors such as age, sex, smoking status, body mass index, nutrition, and education, but it is becoming increasingly evident that genetics may play a role.
Perlee et al developed a CNV prediction model based on the genetic results from the AREDS population with early or intermediate disease. DNA specimens from the AREDS study subjects were genotyped for 14 single nucleotide polymorphisms (SNPs) in genes previously shown to be associated with development of CNV. They found that CNV prediction models that combined both genotype results with phenotypic risk factors improved CNV prediction when compared with phenotypic risk factors alone.7
What about response to treatment in advanced AMD? Why do some patients respond better to anti-VEGF than other patients with similar disease? A number of studies over the past several years have reported the effect of certain CFH polymorphisms on the response to the treatment of AMD with intravitreal anti-VEGF agents. For example, the CC genotype of CFH has been documented to have a poor response in some studies, whereas the TT genotype was associated with a good response to therapy.7-11 However, there are still conflicting studies, so genetic testing is not yet recommended to choose which patients will benefit from intravitreal injections of anti-VEGF.
AREDS formulation (antioxidants and zinc)
Millions of Americans are currently taking the AREDS formulation (antioxidants plus 80 mg zinc) on a daily basis. AREDS, published by the National Eye Institute (NEI) in 2001, was the first study to demonstrate that a combination of vitamin C, beta-carotene, vitamin E, 80 mg of zinc, and 2 mg of copper (the AREDS formulation) reduced risk of progression to advanced disease by 25 percent in intermediate AMD patients.12 For the next 12 years, this was the only formulation recommended by eyecare practitioners, with the exception of a smoker’s formulation without beta carotene because of its association with lung cancer.13
Then, in 2013, the NEI released the results of AREDS2, which examined the benefit of carotenoids (lutein and zeaxanthin); formulations without beta-carotene; and formulations with low-dose (25 mg) zinc on the risk of progression.14 Researchers concluded that carotenoids reduced risk by 18 percent in the sub-group without beta-carotene (presumably because of better absorption of the carotenoids without beta-carotene) and by 20 percent in the sub-group with an original diet poor in carotenoids. In addition, AREDS2 concluded that there was no statistically significant difference between the 80 mg of zinc and 25 mg of zinc in progression to advanced AMD. Despite this finding, the NEI has not made any recommendations to change the current formulation of zinc (80 mg).
AMD supplements may accelerate the disease in some people
There is ongoing controversy clouding the picture for the clinician who seeks to answer whether genetic testing is advised to determine which formulation, if any, intermediate AMD patients should be using and if patients should be on low-dose zinc formulations, considering AREDS2 did not find any difference between the two doses. The concept of using genetic information to tailor treatment, genome-directed therapy, has been gaining momentum over the past few years for a number of systemic diseases. As for AMD, should all patients be on the same AREDS formulation (except for the smokers formula, without beta carotene)? Should some patients be on antioxidants and zinc, or on zinc alone, or on antioxidants alone? Is there value to genome-directed therapy?
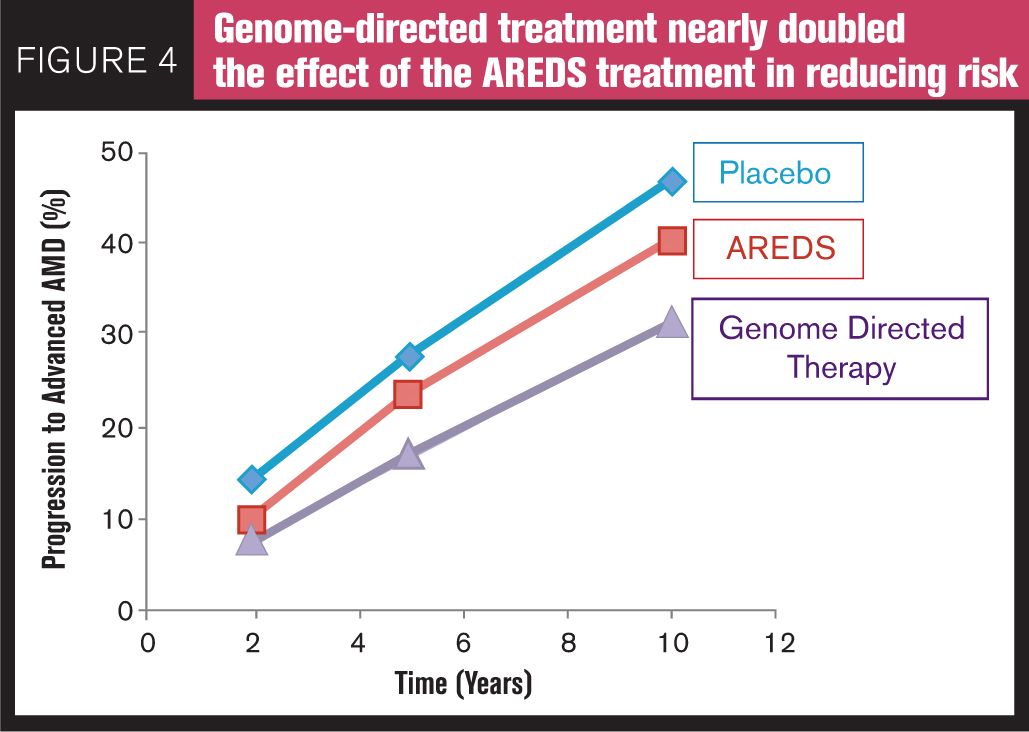
Awh et al15 evaluated a comprehensive set of AMD genetic risk predictors in 995 white patients from the original AREDS study who were in category three disease in one eye and category one, two, three, or four disease in the fellow eye at enrollment. Disease progression was defined as the development of AREDS category four in either eye of patients without category four at enrollment or the development of bilateral category four in patients with unilateral category four at the time of enrollment. Patients were divided into nine groups depending on genotype and treatment group over time. They found significant differences in progression rate for patients having risk alleles in CFH and ARMS2 treated with antioxidants and zinc vs. zinc alone. In their study, they reported that patients with no CFH risk alleles and one or two ARM2 risk alleles benefited most from a zinc-only supplementation, whereas patients with one or two CFH risk alleles and no ARMS2 risk alleles derived maximum benefit from antioxidant-only supplementation-and in these patients treatment with zinc was associated with increased progression to advanced AMD. Dr. Awh stated at the Academy of Ophthalmology Retina Sub-Specialty meeting in November 2013 in New Orleans, LA, that if all of the AREDS patients were treated with genotype-directed therapy, the reduction in the 10-year progression to advanced AMD could potentially be 33 percent with the genotype-directed therapy vs. only 14 percent with the AREDS formulation (Figure 4). The authors of the study have publicly disclosed a commercial relationship with ArcticDx, Inc., one of the two companies that perform genetic testing.
Following this publication, Chew et al16 analyzed the same patient data using an alternative statistical approach in which they did not find any association between CFH and ARMS2 genotypes and response to the AREDS formulation, which refuted Awh’s data. However, scrutiny of their statistical model by Awh et al in a second publication6 revealed that because patients in Chew’s study were divided into 27 subgroups, it was underpowered to refute the data of the original Awh study.
Addressing AREDS2 controversies
What about the dosage of zinc? Recall that the AREDS formulation contains 80 mg of zinc, which is eight times greater than the recommended daily allowance. Because 80mg is a high dose of zinc, AREDS2 looked at any statistically significant differences in progression between patients using 80 mg of zinc versus the group using 25 mg of zinc and found that there were none; in other words, a patient benefiting from the AREDS formulation would get the same benefit (lower risk to AMD progression) if that formulation contained only 25 mg of zinc. Then why should a patient take 80 mg of zinc when he could gain the same benefit from 25 mg of zinc? High doses of zinc have been associated with a significant increase in genitourinary problems, some of which have required hospitalizations.17 High doses of zinc can cause GI upset and have been implicated in Alzheimer’s disease.18 High levels can interfere with the absorption of some antibiotics19 and may decrease high-density lipoproteins, the so-called “good cholesterol.”20 The NEI has not, as yet, suggested making any modifications in the AREDS formulation containing 80 mg of zinc. The clinical director of the NEI has publicly acknowledged that he and others have a patent on the formulation containing 80 mg of zinc but not on 25 mg of zinc. So, what about the intermediate AMD patient whose genetic profile suggests a recommendation free of zinc but the patient is taking the AREDS formulation with 80 mg of zinc? Should that patient remain on that formulation? Other formulations are available (Table 2).
Lutein and zeaxanthin
In AREDS2, in the overall group, no benefit was found in patients who took lutein and zeaxanthin in reducing the risk of progressing to advanced AMD. However, two subgroups were later identified which did benefit-those who took lutein and zeaxanthin with no beta carotene (18 percent reduced risk of progression) and those who had low amounts of lutein and zeaxanthin in their diets (median 0.7 mg per day). That sub-group experienced a 26 percent risk reduction.14
Does genetic testing yet play a role in determining who will respond to lutein and zeaxanthin supplementation? Yonova-Doing et al21 recently demonstrated an association between SNPs in SCARB1, RPE65, ABCA1, and FADS1 and response to carotenoid supplementation (MPOD response) and ELOV12 and changes in lutein concentration. These genes code for proteins affecting carotenoid transport and fatty acid metabolism, and hence SNPs or variants in these genes might determine whether or not patients would benefit from carotenoid supplementation. These genes are currently not included in the commercially available tests but may be in the future.
Conclusion
Genetic testing for the AMD high-risk alleles is available. But how and when should it be used? Should it be used at all? In 2012, a task force from the American Academy of Ophthalmology issued a recommendation22 to avoid “routine” genetic testing for genetically complex disorders like AMD until specific treatments are shown in published clinical trials to be of benefit. This could take years. Individual practitioners should decide whether their patients with intermediate AMD and/or who have drusen and a high number of risk factors who are not “routine” would benefit now from genetic testing. Practitioners must continue to read the literature and keep abreast of new developments in the field. Recommendations for specific nutritional supplementation in this category of patients depending on the patient’s CFH and ARMS2 alleles are available for practitioners who may be concerned about the intermediate AMD patient on formulations containing high concentrations of zinc. One can wait until specific treatments are shown in published clinical trials to be of benefit and wait for studies from authors who are free of personal conflicts and statistical interpretative issues or decide now if some patients may benefit from this testing. The decision is up to individual practitioners at this point in time. However, most experts who have weighed in on the subject agree on one thing: genetic testing for the prognosis and treatment of AMD will play a role in the management of the AMD patient. The question is when. Will it be now for some and in the future, for others?
Check out our three-part guest perspective conversation on mesozeaxanthin:
Part 1: The third carotenoid-mesozeaxanthin (Z-RS) and who needs to consume it
Part 2: Rebutting mesozeaxanthing findings
Part 3: Continuing the conversation on mesozeaxanthin
References
1. National Eye Institute. Prevalence of Age-Related Macular Degeneration In the United States (NEI Statistics and Data). Available at http:/www.nei.nih.gov/eyedata/amd.asp. Accessed June 6, 2014.
2. AMD Alliance International. Basic facts about AMD. Available at: http://www.amdalliance.org/information_overview_basic_facts.html. Accessed June 5, 2014
3. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005 Apr 15;308(5720):419-21.
4. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005 Apr 15;308(5720):385-9.
5. Edwards AO, Ritter R III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005 Apr 15;308(5720):421-4.
6. Awh CC,Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study (published online ahead of print September 4, 2014) Ophthalmology 2014. Doi:10.1016/j.ophtha.2014.07.049.
7. Perlee LT, Bansal AT, Gehrs K, et al. Inclusion of Genotype with Fundus Phenotype Improves Accuracy of Predicting Choroidal Neovascularization and Geographic Atrophy. Ophthalmol. 2013 Sep;120(9):1800-92.
8. Dikmetas O., Kadayfocular S, Eldem B. The effect of CFH polymorphisms on the response to the treatment of age-related macular degeneration (AMD) with intravitreal ranibizumab. Mol Vis. 2013 Dec 20;19:2571-8.
9. Kloeckener-Gruissen B, Barthelmes D, Labs S. Genetic association with response to intravitreal ranibizumab in patients with neovascular AMD. Invest Ophthalmol Vis Sci. 2011 Jul 1:52(7):4694-701
10. Abedi F, Wickremasinghe S, Richardson AJ, et al. Genetic influences on the outcome of anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Ophthalmol. 2013 Aug;120(8):1641-8.
11. Micieli JA. Can genetic factors predict response to antivascular endothelial factor therapy in age-related macular degeneration? Can J Ophthalmol. 2011 Dec;46(6):549-51.
12. Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
13. Omenn GS, Goodman GE, Thornquist MD et al. Risk factors for lung cancer and for intervention effects in CARET, The Beta-Carotene and Retinol Efficacy Trial. J. Natl Cancer Inst. 1996;88:1550-9.
14. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study (AREDS2) randomized clinical trial. JAMA. 2013 May 15; 309(19):2005-15.
15. Awh CC, Lane A, Hawken S, et al. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmol. 2013 Nov;120(11):2317-23.
16. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS Report Number 38. Ophthalmology 2014. Jun 26. pii: S0161-6420(14)00428-X. doi: 10.1016/j.ophtha.2014.05.008. [Epub ahead of print]
17. Johnson AR, Munoz A, Gottlieb JL, et al. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007 Feb;177(2):639-43.
18. Cuajungco MP, Lees GJ. Zinc and Alzheimer’s disease: is there a direct link? Brain Res Rev. 1997 Apr;23(3):219-36.
19. Lomaestro BM, Bailie GR. Absorption interactions with fluoroquinolones. 1995 update. Drug Saf. 1995 May;12(5):314-33.
20. Hooper PL, Visconti L, Garry PJ, et al. Zinc lowers high-density lipoprotein-cholesterol levels. JAMA. 1980 OCt;244(17):1960-1.
21. Yanova-Doing E, Hysi P, Venturini C, et. al. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp Eye Res. 2013 Oct;115:172-7.
22. Stone EM, Aldave AJ, Drack AV, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthalmology 2012;119:2408-10.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.