FDA approves first small aperture IOL for cataract surgery
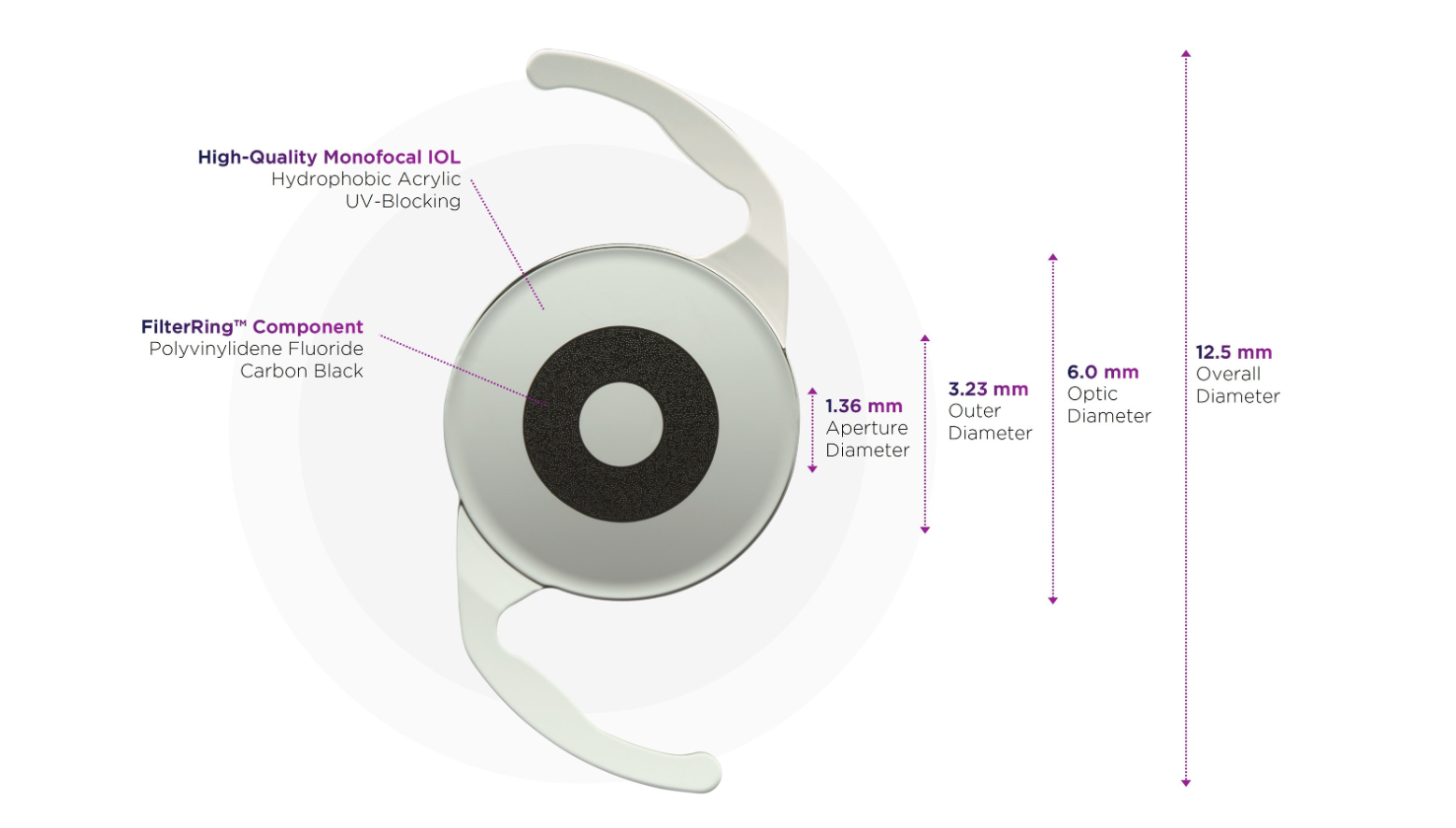
AcuFocus's IC-8 Apthera IOL features a proprietary small aperture technology that filters out peripheral defocused light and allows only focused light to reach the retina.
The IC-8 Apthera IOL is the first lens indicated for implantation with a monofocal or monofocal toric IOL in the fellow eye, the first EDOF lens indicated for monovision, and the first non-toric IOL indicated for cataract patients with low amounts of corneal astigmatism. Image courtesy of AcuFocus.
AcuFocus announced Monday that the US FDA approved its IC-8 Apthera intraocular lens (IOL) for cataract treatment.
As the first—and currently only— non-toric extended depth of focus (EDOF) IOL, the lens features a proprietary small aperture technology that filters out peripheral defocused light and allows only focused light to reach the retina, according to a company news release.
Approval of the Apthera IOL follows recent data from the US Investigational Device Exemption 12-month study of 453 participants that analyzed the safety and efficacy of the IOL implanted in one eye and a monofocal or monofocal toric IOL implanted in the second eye.
Outcomes for the Apthera IOL group (343 participants) were compared to a control group (110 participants) who received a monofocal or monfocal toric IOL in both eyes.
Those with Apthera IOL-treated eyes maintained a 2 D of EDOF and demonstrated 0.91 D of additional range of vision benefits over monofocal IOL eyes at 0.2 logMAR threshold, exceeding the 0.50 D ANSI criterion for EDOF IOLS, according to the release.
Further, those participants with Apthera IOL-treated eyes also reached an equivalent uncorrected distance vision and statistically superior intermediate and near vision in comparison to the control group participants.
Additional achievements of the Apthera IOL group included comparable binocular contrast sensitivity to control subjects in both photopic and mesopic conditions—which was a first reported for an EDOF lens, the release stated.
“The Apthera IOL represents several firsts for surgeons and patients: the first small aperture IOL to receive FDA approval, the first lens indicated for implantation with a monofocal or monofocal toric IOL in the fellow eye, the first (EDOF] lens indicated for monovision, and the first non-toric IOL indicated for cataract patients with low amounts of corneal astigmatism,” stated AcuFocus CEO and President Al Waterhouse, the release.
A limited commercial release of the Apthera IOL in the US is expected in fall 2022.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.