FDA clears C. Light to market retinal eye movement monitor, Retitrack™
This medical device is the first retinal eye-movement monitor for non-invasive, objective clinical assessments.
Photo courtesy of C. Light Technologies, Inc.
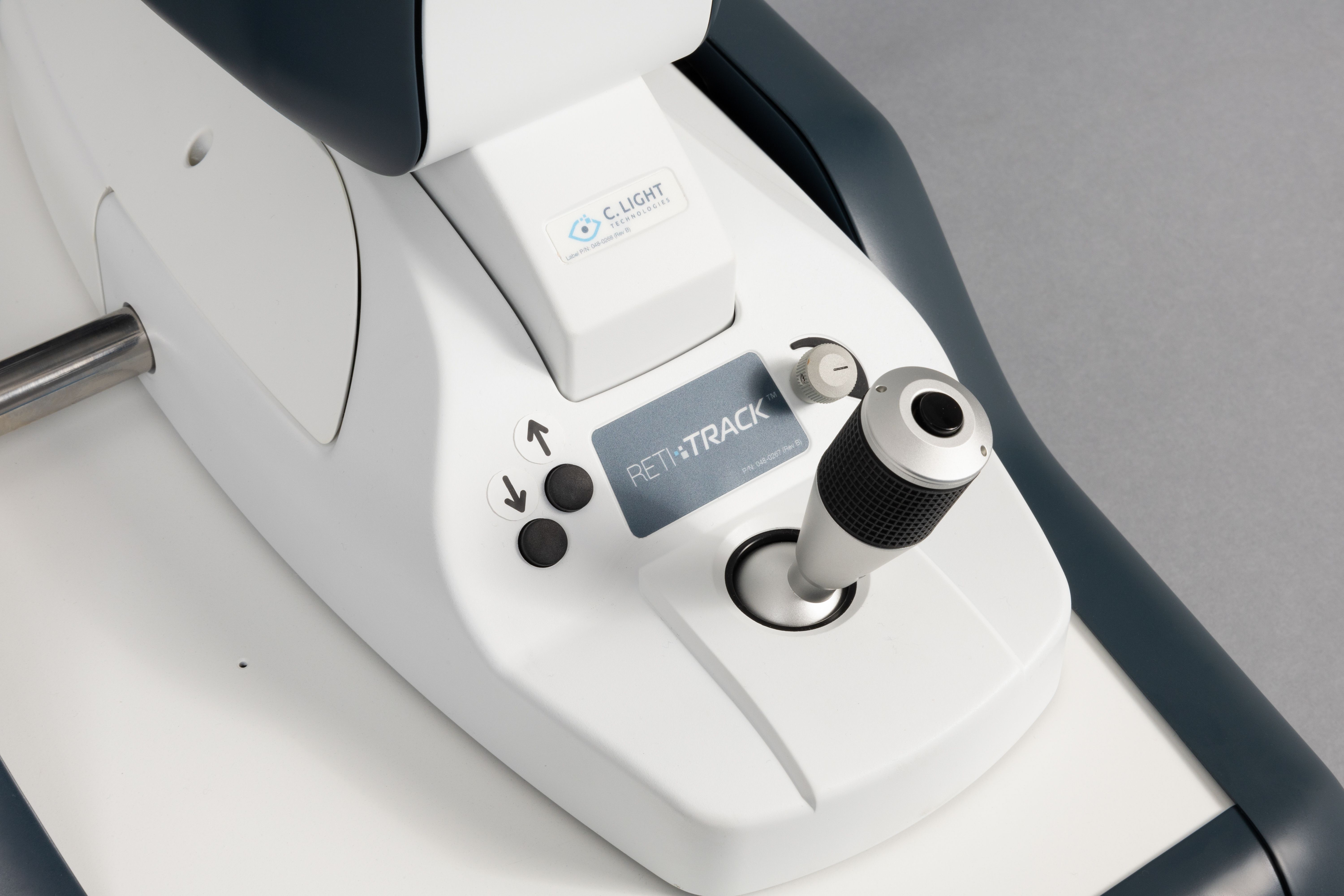
The U.S. Food and Drug Administration (FDA) cleared the Retitrack by C. Light Technologies, Inc., for marketing through premarket notification 510(k). The Retitrack device will provide a new tool to better understand oculomotor function via the retina, providing concrete and objective metrics to medical professionals.
The Retitrack is a monocular, tabletop eye movement monitor that is intended for recording, viewing, measuring, and analyzing temporal characteristics of fixation and saccadic responses when viewing a visual stimulus and is intended for use by healthcare practitioners within the healthcare setting (e.g., physician's office, clinic, laboratory).
Photo courtesy of C. Light Technologies, Inc.

The Retitrack
The Retitrack stands alone as the first retinal eye-movement monitor cleared for use within the healthcare field. The device operates by recording 10-second, high-resolution retinal videos at the photoreceptor level, allowing for quantification of eye motion down to 0.1 degrees. Its accompanying software extracts and analyzes both fixation (microsaccades and drift) and saccadic eye movements in real time and generates a comprehensive summary report for clinical interpretation.
"Fixational eye movements have previously eluded clinical quantification, posing a significant challenge to healthcare professionals who are aiming to improve prognostic care. With our novel technology, we've unlocked the potential of one of the smallest motor movements in the human body, offering invaluable data that will drive the future of clinical care," said Christy Sheehy-Bensinger, PhD, CEO and co-founder.
"We are gratified that our device is cleared for marketing and that we hit our seed funding milestone. Our device and its accompanying software will revolutionize healthcare by empowering clinicians with significant new insights into an individual's oculomotor function, as opposed to existing devices reporting on retinal structure alone," Sheehy-Bensinger continued.
"C. Light fills a critical need in research and the clinic. We're very excited by how quickly this team has achieved their major milestones. C. Light will have a significant, lasting impact on the field in coming years," said lead investor James Wang, Creative Ventures.
"I am proud of our team. We meticulously operate at a high standard to propel our tech development forward and ensure our product is scientifically well-conceptualized and anchored in precision and accuracy," said Joe Xing, PhD, CTO and co-founder.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.