Genetic testing: Simplifying the diagnoses of inherited retinal diseases
During a presentation at the American Academy of Optometry 2021 annual meeting in Boston, Sherry Bass, OD, FAAO, described for attendees the circuitous path that clinicians have had to negotiate to establish a diagnosis in patients with inherited retinal diseases, how that path has become more straightforward, and examples of how genetic testing can help in the diagnosis of IRDs in tricky cases.
Reviewed by Sherry J. Bass, OD, FAAO
The circuitous path that clinicians have had to negotiate to establish a diagnosis in patients with inherited retinal diseases (IRDs) has become more straightforward, at during a presentation at the American Academy of Optometry 2021 annual meeting in Boston, Sherry Bass, OD, FAAO, Distinguished Teaching Professor at SUNY State College of Optometry in New York, provided some examples of how genetic testing can help in the diagnosis of IRDs in tricky cases. Here are some of the highlights.
Genetic testing
In the past, the methods of DNA sequencing used to be very expensive and slow. During this process, a physician obtained a blood sample and forwarded it to a testing laboratory, which made it difficult for optometrists to order genetic testing.
In 2021, the scenario is dramatically different—genetic testing for optometrists is simple, fast, and free if the patient meets certain criteria, which include at least one of the following: a) night blindness b) central vision loss c) deterioration of color vision loss d) peripheral field loss and e) photophobia. Patients with AMD and/or oculocutaneous albinism are excluded. The clinician can open a test kit, fill out a form, have the patient sign a consent form, obtain a buccal sample, label the sample, and mail the sample using the envelope and mailing label provided in the kit. Free test kits for IRDs are available at Invitae.com and My Retinal Tracker.com
The clinician will receive an email confirmation when the sample is received and the sample will be assigned an identification number. If there is a problem with the sample such as insufficient or degraded genetic material , the clinician will be notified by email. Some potential problems to avoid are unlabeled tubes, unsigned consent forms, or missing information on the form.
The results of the genetic analysis generally take 4 to 6 weeks to arrive by email.
A puzzling case: retinitis pigmentosa (RP) or not?
Bass provided a representative case of a 58-year-old man who underwent an ocular examination to procure a new prescription for reading glasses. He denied previous ocular or systemic health problems. His best-corrected visual acuity (BCVA) was 20/20 bilaterally at distance and near with correction for presbyopia. While undergoing a gross confrontation visual field examination, he remarked that superiorly he was able to see the examiner’s fingers but not the fingertips.

Retinal examination revealed symmetric inferior arcuate areas of what appeared to be retinal and choroidal degeneration (Figure 1). Additional testing, including visual fields, revealed bilateral mirror-image dense superior arcuate field defects (Figure 2). An electroretinogram (ERG) was performed which was normal. The patient denied any complaints of night vision (nyctalopia) or visual field difficulties.
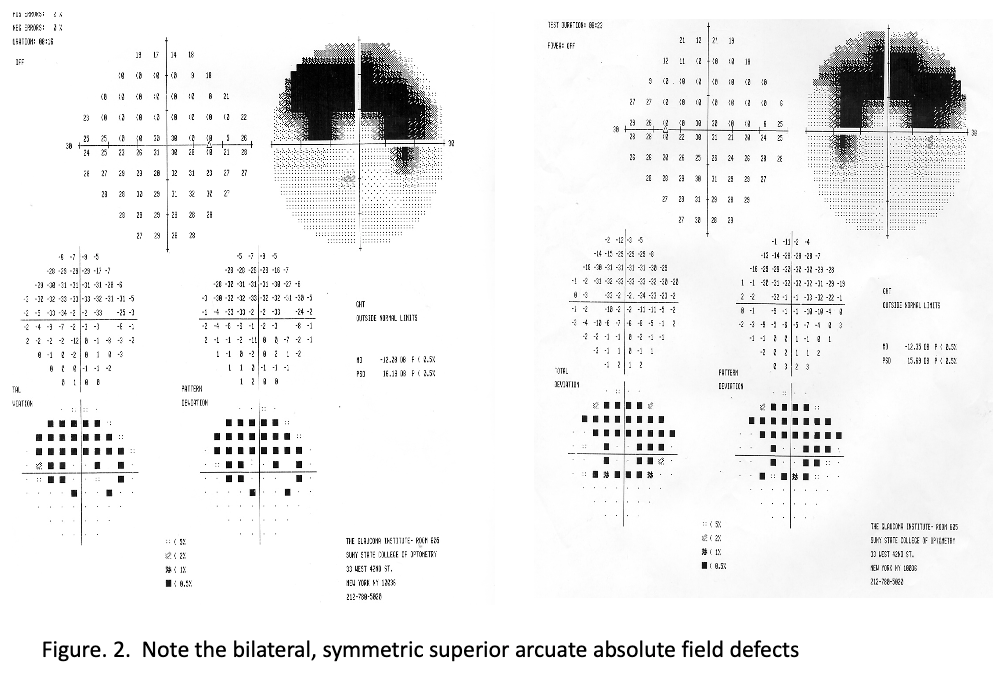
The results begged the question about whether this was a known disease variant or a new disease altogether, Bass said.
A look into the ocular findings in close relatives found that his 62-year-old brother was symptomatic with a long-standing history of a retinal problem that appeared as a stable ring of retinal and choroidal degeneration without nyctalopia. The BCVA was 20/25 bilaterally. The ERGs showed reduced scotopic and photopic recordings. The patient’s 37-year-old son was asymptomatic with BCVAs of 20/20 bilaterally. A 33-year-old niece had unexplainable visual field loss for over 10 years (accidentally discovered when she had a visual field performed on her as a medical student) with bilateral BCVAs of 20/20. The patient’s deceased father reported at 53-years of age that he had difficulty with near work. The distance BCVAs were 20/25+ and the near was J2 bilaterally. Although no photos were available, the eye doctor diagnosed the patient with an “old chorioretinitis in both eyes” in the papillomacular area and there was a relative paracentral scotoma with central and peripheral sparing.
The patient’s diagnosis remained unclear. Bass questioned if this was a form of RP, but key components were missing. There was no nyctalopia, no arteriolar attenuation, no progression to blindness, or peripheral field loss. Choroidal atrophy was present not just retinal degeneration. The ERGs were normal in all affected family members except that of the most affected member.
After a 3-month wait time, genetic testing identified a pathogenic variant in the RHO gene of the patient and a diagnosis of autosomal-dominant RP was established.
Advances in LCA
Leber congenital amaurosis (LCA), an autosomal recessive disorder, is characterized by severely reduced vision at birth, nystagmus, and a nonrecordable ERG. The fundus can look like anything and is not necessarily helpful in the diagnosis.
A novel gene therapy, voretigene neparvovec (Luxturna, Spark Therapeutics, Inc.), was FDA-approved in December 2017 to treat LCA patients who have bi-allelic (homozygous) variants in the RPE65 gene. It requires a sub-retinal injection and uses a virus-vector to transport a normal copy of the RPE65 gene into the retina. The cost is $425,000 per eye, but it is a “one and done” injection.
Bass reported the case of a 23-year-old man with LCA characterized by nystagmus and a nonrecordable ERG, who was the second such patient tested at SUNY for the RPE65 variant. The right eye BCVA was no light perception due to an old retinal detachment and the left eye BCVA was 20/320.
Genetic testing showed that the patient was homozygous in the RPE65 gene for a variant, c.1022T>C, which results in the amino acid substitution p.Leu341Ser, previously reported in patients with retinal disorders. The result was consistent with LCA2. The patient’s examination showed that the central and near midperipheral retinal structure was relatively preserved, which made him a candidate for a potential gene augmentation treatment, such as Luxturna, in the left eye.
Bass emphasized the importance of establishing patient expectations, noting it is always best to be realistic.
“Patients can expect to see better in dim light and at night, when vision will be ‘brighter,’” she said. “Nystagmus may be relieved a bit and better VA achieved as a result. Patients over 20 years may not achieve the same good results as younger patients because of more advanced disease.”
Bass also advised that insurance approval of the treatment can take months in some cases. The treatment is not a cure and the disease is still progressive.

The current patient reported brighter night vision and a brighter left visual field after treatment (Figure. 3) All he wanted to do was to be able to see better when he walked his dog at night.
A tricky diagnosis
A 12-year-old patient with refractive amblyopia was referred for evaluation of a “retinal vasculitis without vitritis” (Figure 4); the BCVAs were 20/30 bilaterally secondary to refractive amblyopia. The etiology of the inflammation was unclear and the patient was referred to our retina clinic.

Additional imaging revealed autofluorescence changes in the outer retina (Figure 5) that are not indicative of vasculitis, but more indicative of an IRD. In addition, the OCTs revealed loss of the photoreceptor integrity line (ellipsoid zone) and thinning of the RPE (Figure 6). Was this case vasculitis really a form of RP?, Dr. Bass asked. Additional questioning of the patient uncovered difficulty with night vision. The ERG rod and cone amplitudes were reduced by 30%, greater in the left eye.

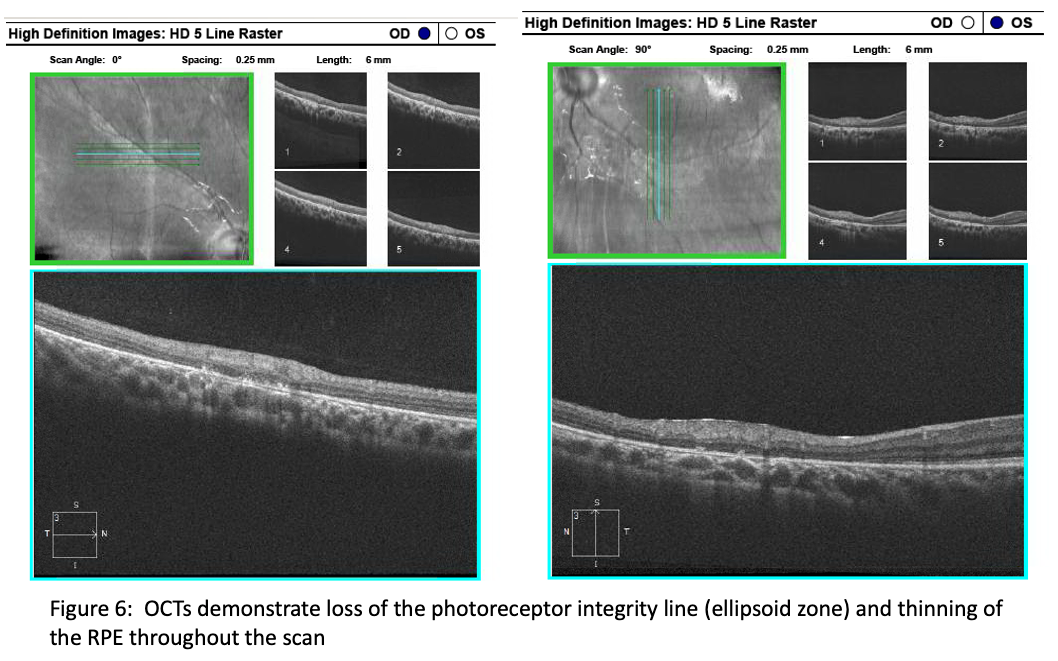
Genetic testing identified a pathologic variant in AGBL5, which is associated with autosomal recessive RP, not vasculitis, which is a different disease, requiring extensive blood testing to determine the etiology.
If you are not comfortable discussing the results with patients, the genetic testing offers the services of free genetic counseling.
A take-home message for optometrists is that genetic testing is a dynamic process. She explained that variants of uncertain significance may become certain over time as more DNA is analyzed. With the introduction of more information into the genetic mix, results change. Amended reports are sent if there are any such changes.
“Genetic testing is now available to optometrists,” Bass concluded. “It is free if the patient meets certain criteria. It is easy to perform, and fast. Consider genetic testing for patients where the phenotypic presentation is not diagnostic, for patients who are interested in on-going clinical trials, in cases where treatment may be available and/or for family planning.”
Sherry Bass, OD, FAAO
This article is adapted from Bass’s presentation at the American Academy of Optometry 2021 annual meeting in Boston. She has no financial interest in this subject matter.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.