How tear osmolarity affects lens wear
Considering that many CL patients are desensitized, it is critical we not only monitor their tear film and ocular surface for change, but we demonstrate stability or instability through measurable means. This creates trust, validates our recommendations, induces compliance, and generates positive outcomes.

We have heard it a million times: Discomfort is the number one reason that patients drop out of their contact lenses (CL).1 Yet, when we sit behind the slit lamp explaining ocular surface findings that will lead to discomfort, the patient refuses to take the warning seriously.
A challenge for many of us is that CL patients perpetuate their wearing behaviors based on the perception of past success, or lack of obvious consequence. These patients believe if what they are doing has not hurt them by now, then it never will.
The conundrum continues because it is these patients who end up dropping out of CL wear without notice.
For many of us, our CL volume remains stable year after year. This may seem encouraging, but it is actually a negative discovery. It means our CL practice is essentially a revolving door. Though we may not remember many patients quitting CLs this year, we know we have had many new fits.

Think about it for a minute.
Previously from Dr. Brimer: 9 habits for multifocal contact lens fitting success
If we have continuous new fits, yet our CL volume is steady, then we have as many patients leaving CL wear, or the practice. This can have a significant financial impact over the life of the practice, resulting in $19,000 to $24,500 in total lost revenue per CL dropout.1
Considering that CL dropouts often don’t give us notice or the chance to fix their situation, are there tools we can implement to predict future CL intolerance? Osmolarity testing can be one tool used.
What osmolarity tells us
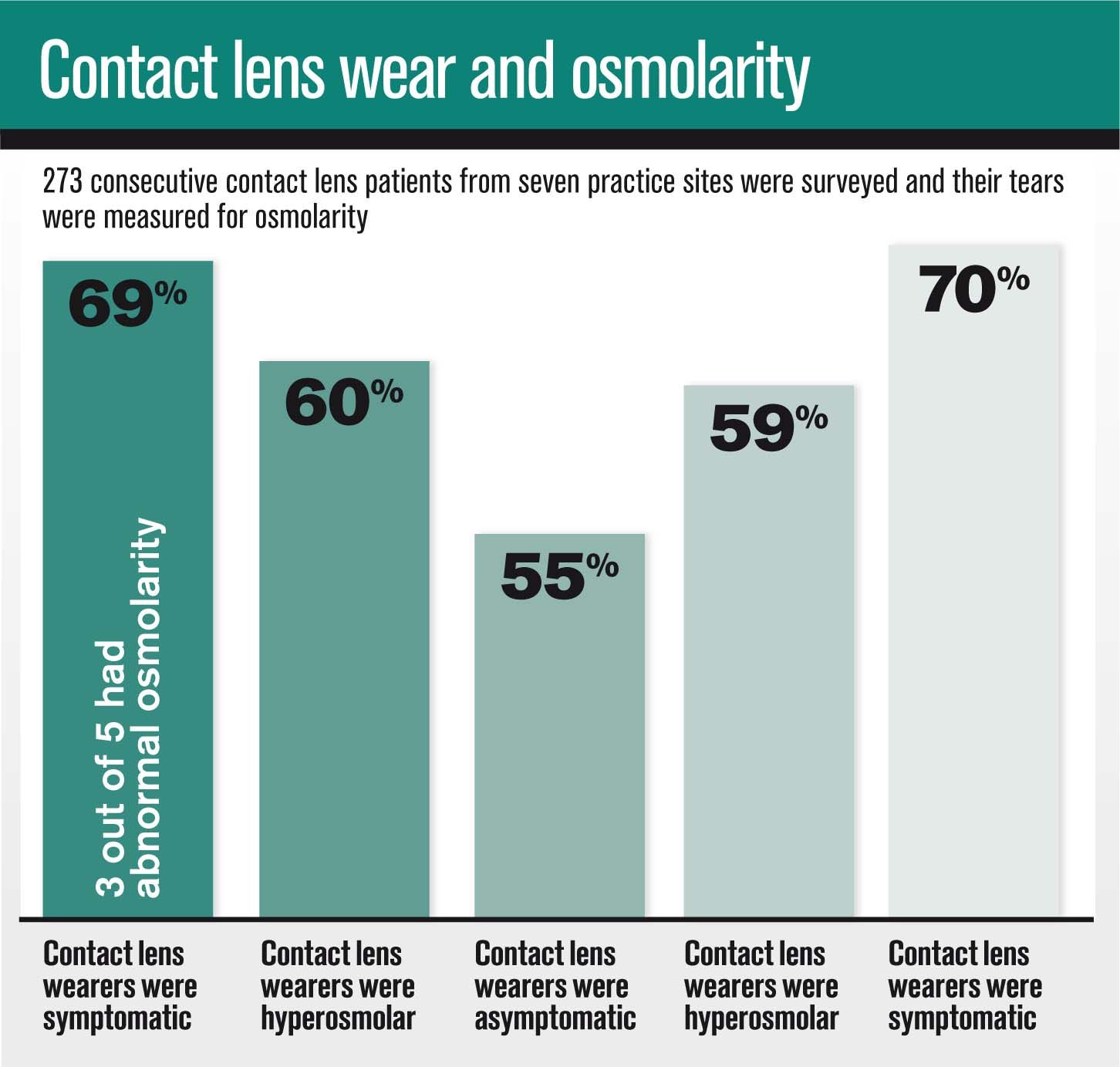
I recently participated in a clinical trial in which 273 consecutive CL patients from seven practice sites were surveyed and their tears were measured for osmolarity. Patients were categorized as “symptomatic” if reporting more than three of the 10 potential symptoms. Abnormal osmolarity was defined as a difference of greater than 8 mOsml/L between both eyes or either eye testing over 308 mOsml/L.2
Related: Sizing up daily disposable contact lenses
First, let’s consider what osmolarity tells us. Symptoms of CL discomfort can mimic dry eye disease. A CL splits the tear film in half, creating a pre-lens and post-lens tear film. This causes an increase in tear film evaporation and, in some cases, CL induced dryness.
Osmolarity measures the concentration of solutes in the tear film. The more concentrated the tear film, the higher the osmolarity. Tears become hyperosmolar when there is a decrease in aqueous production or increased evaporation due to decreased meibomian gland production.
In this state, tears can become toxic to the corneal epithelium. Toxicity can then lead to a loss of glycocalyx and microvilli, which are responsible for attracting and retaining water on the corneal surface. Instability of the tear film and lack of wettability can cause epithelial cell damage, goblet cell loss, inflammation, and even cell death.
Nerve fibers and underlying cells are then exposed, and damage progresses. As surface wettability decreases, it affects the way light is reflected, causing increased aberrations and visual strain for the patient. This is amplified in those wearing CLs, often inducing fluctuating vision that varies with the blink, or the need to blink more frequently.
How osmolarity is applicable
When surveyed, 69 percent of all CL wearers in the study were symptomatic. This begs the question: Are we asking the right questions in the exam room to uncover that potentially 69 percent of our wearers are symptomatic?
Ask specific questions to better screen your patients. Don’t wait for them to complain!
Of these symptomatic patients, three out of five had abnormal osmolarity. As expected, a higher percentage of CL wearers were hyperosmolar in the symptomatic group vs. the asymptomatic group, but there was not a big differential between the two groups (60 percent vs. 55 percent).
Related: Connecting allergy and osmolarity
This is a reminder to us of the impact of contact lens wear to the ocular surface, even in patients without current symptoms.

Research shows multiple inflammatory mediators, such as interleukin IL-6 and IL-8, are upregulated in the tears of CL wearers.3 It also has been shown that both tear break-up time (TBUT) and tear volume are significantly reduced in CL wearers. It seems plausible that osmolarity would be increased in a significant portion of CL wearers, regardless of their symptoms.4
Interestingly, of the 59 percent who tested hyperosmolar, 70 percent of those were surveyed to be symptomatic. It would be beneficial to know if and when the other 30 percent become symptomatic.
With that in mind, perhaps osmolarity can be used as a meaningful marker in determining the tear film balance, thereby predicting the likelihood of future discomfort and potential dropout. It would be helpful to identify any increase in osmolarity over time. If a patient is asymptomatic but hyperosmolar, watch for progression and/or future onset of symptoms.
Consider being proactive and implementing solution, material, modality, or behavioral changes now, as well as some form of treatment. Discuss diet, water intake, wearing habits, and simple lid hygiene at the least.
The number of symptoms reported and frequency of the symptoms was similar among patients with normal and abnormal osmolarity. This indicates that symptoms alone may not be an adequate indicator of ocular surface stress.
Related: How to use tear osmolarity to help treat dry eye disease
So, if a large percentage of CL wearers are symptomatic, how do we know which ones might progress and drop out?
Determining dropout patients
It is reasonable to assume that patients with more concentrated tears might progress to a state of hydrophobicity and quickly evolve to experience greater discomfort and structural consequence.
If a patient is symptomatic and hyperosmolar, implement a treatment now to prevent progression. Also, consider an immediate change to the patient’s material, solution, or wearing habits. Use osmolarity as a conversation starter to motivate patients to convert to daily disposable CLs.
If your patient is symptomatic with normal osmolarity, look for another source for his discomfort. Contributors might include hypersensitivities, allergies, blepharitis, partial blinks, lagophthalmos, conjunctival chalasis, ocular misalignment, or other visual disturbance.
If your patient is asymptomatic with normal osmolarity, celebrate! Not only can osmolarity be useful in identifying problems in the tear film and ocular surface, but osmolarity can be a valuable marker in determining if changes made in solution or material are truly positively affecting the ocular surface.
Once osmolarity returns to normal, you can offer the patient encouragement that symptomatic relief will likely follow. This translates to increased comfort and wear time for the patient and offers ODs peace of mind that we are being proactive to ensure long-term CL success and fewer dropouts.
Related: Ocular surface disease limits surgical options
Measurable markers key to success
The message you are attempting to deliver to the patient will be better received and lead to better compliance when you use markers that are measurable. Along with osmolarity, I use the Crystal Tear Report on the Oculus 5M Keratograph to show the patient any debris floating in the tear film, tear meniscus height, tear break-up time, partial blinks, redness, and the presence or absence of oil.
Tracking how patients’ CL wear impacts these markers helps to validate the conversation to initiate a treatment, change wearing habits, or upgrade the lens itself. By utilizing these markers, I can perform a repeat assessment at a later date to show positive or negative progression.
Considering that many CL patients are desensitized, it is critical we not only monitor their tear film and ocular surface for change, but we demonstrate stability or instability through measurable means. This creates trust, validates our recommendations, induces compliance, and generates positive outcomes.
References
1. Rumpakis J. New Data on Contact Lens Dropouts: An International Perspective. Rev Optom. 2010 Jan;147(1):37-42.
2. Bowling E, Bloomenstein M, Gaddle I, Clay G, Harrell M, Ward J, Brimer C. Prevalence of abnormal tear film quality and stability measured by abnormal tear osmolarity among contact lens wearers. Poster presented at: American Academy of Optometry; Anaheim, Calif.; Nov. 8-12, 2016.
3. Craig JP, Willcox MD, Argüeso P, Maissa C, Stahl U, Tomlinson A, Wang J, Yokoi N, Stapleton F; members of TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Invest Ophthalmol Vis Sci. 2013 Oct 18;54(11):TFOS123-56.
4. Glasson MJ, Stapleton F, Keay L, Sweeney D, Willcox MD. Differences in clinical parameters and tear film of tolerant and intolerant contact lens wearers. Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5116-24.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.