Identifying common macular conditions with OCT
Effectively manage patients through diagnosis and interpretation

As baby boomers age, clinicians need to be comfortable with diagnosing common macular conditions and interpreting optical coherence tomography (OCT) findings. OCT is a diagnostic imaging technique that allows for the qualitative (morphology and reflectivity) and quantitative (thickness, mapping, and volume) analysis of the retinal landscape.1
Patients will rely on you to effectively manage their conditions through diagnosis and interpretation as well as to refer them to a retinal specialist when needed.
The following are the most common macular conditions and their supporting OCT findings.
Read more from Dr. Fludder here
Dry age-related macular degeneration
Dry age-related macular degeneration (AMD) begins with characteristic yellow deposits of extracellular material (drusen) in the macula between the retinal pigment epithelium (RPE) and the underlying choroid. Most people with these early changes still have good vision.
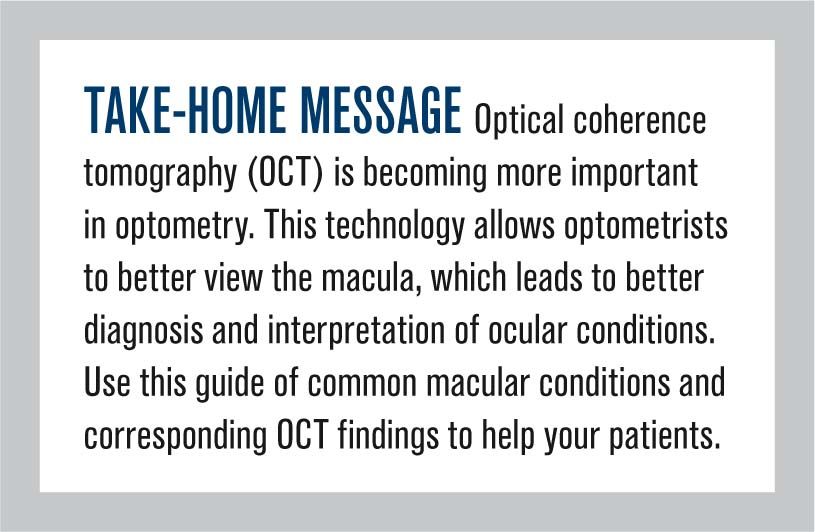
People with drusen may or may not develop AMD. Many people over age 55 have drusen with no negative effects. The risk of developing symptoms is higher when the drusen are large, numerous, and associated with disturbances in the RPE layer. Large and soft drusen are thought to be related to elevated cholesterol deposits.
Although advanced age and family history are the primary risk factors, other factors include:
• Smoking
• High cholesterol
• Fat intake
• Abdominal obesity
• Ultraviolet light exposure
Advanced dry AMD showing central geographic atrophy results from atrophy of the RPE layer that causes vision loss through loss of photoreceptors in the macula.2 Most patients with dry AMD can be effectively managed with education regarding risk factors, vitamin supplements, at-home Amsler grid usage, smoking cessation, and regular dilated exams with OCT.
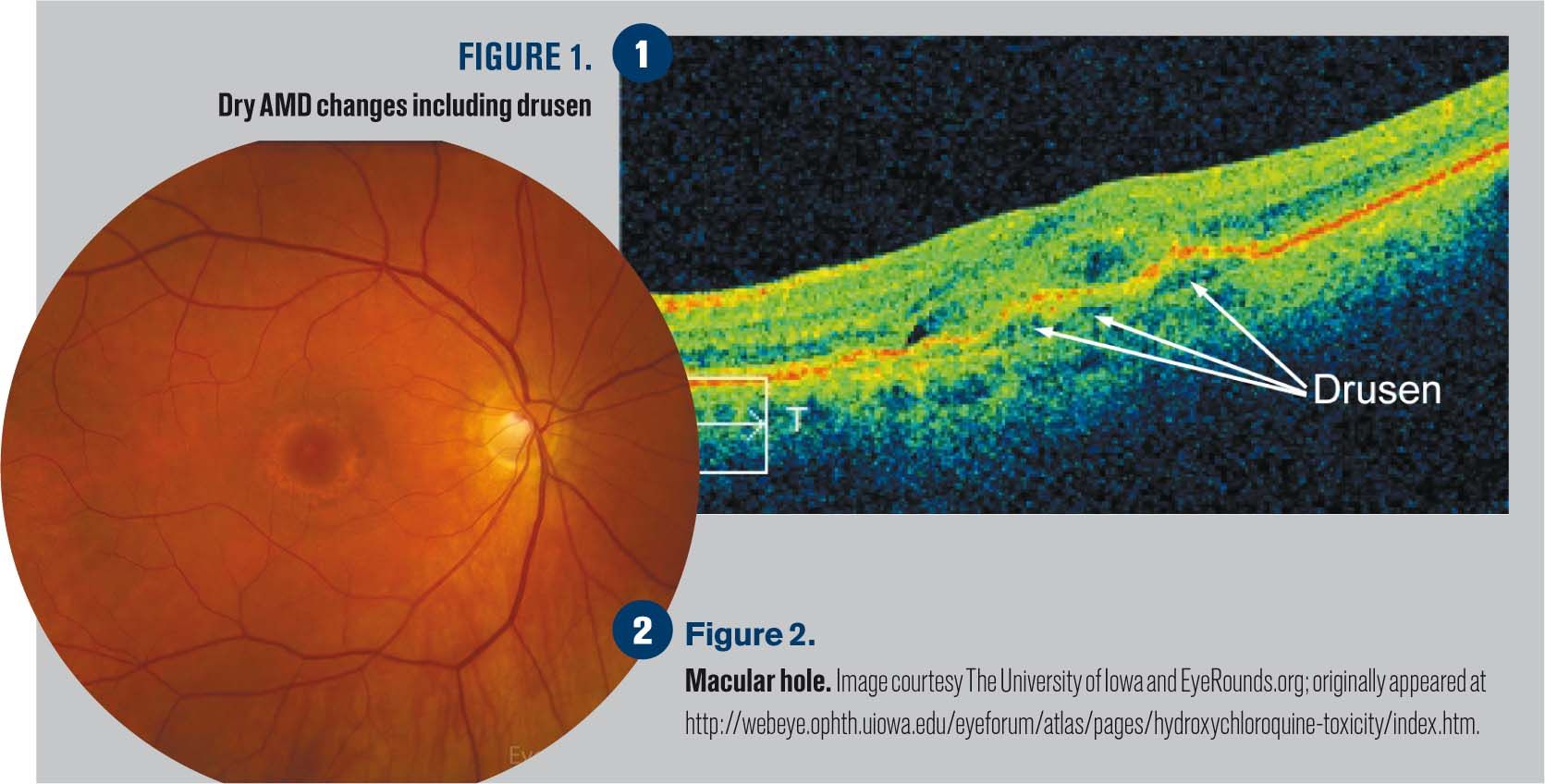
Figure 1 shows dry AMD changes including drusen, which is shown by small elevated disruptions at the level of the RPE and photoreceptors.
Related: Misdiagnosing macular degeneration
Wet age-related macular degeneration
Neovascular or exudative AMD-the wet form of advanced AMD-causes vision loss due to abnormal blood vessel growth called choroidal neovascularization in the choriocapillaris through Bruch's membrane. The proliferation of abnormal blood vessels in the retina is stimulated by vascular endothelial growth factor (VEGF).
Unfortunately, these new vessels are fragile, ultimately leading to blood and protein leakage below the macula. Bleeding, leaking, and scarring from these blood vessels eventually cause irreversible damage to the photoreceptors and rapid vision loss if left untreated.2
Patients should be referred to a retinal specialist for treatment options when exudative AMD is suspected.
Figures 2-4 show wet AMD revealing macular edema as well as choroidal neovascularization.
Cystoid macular edema
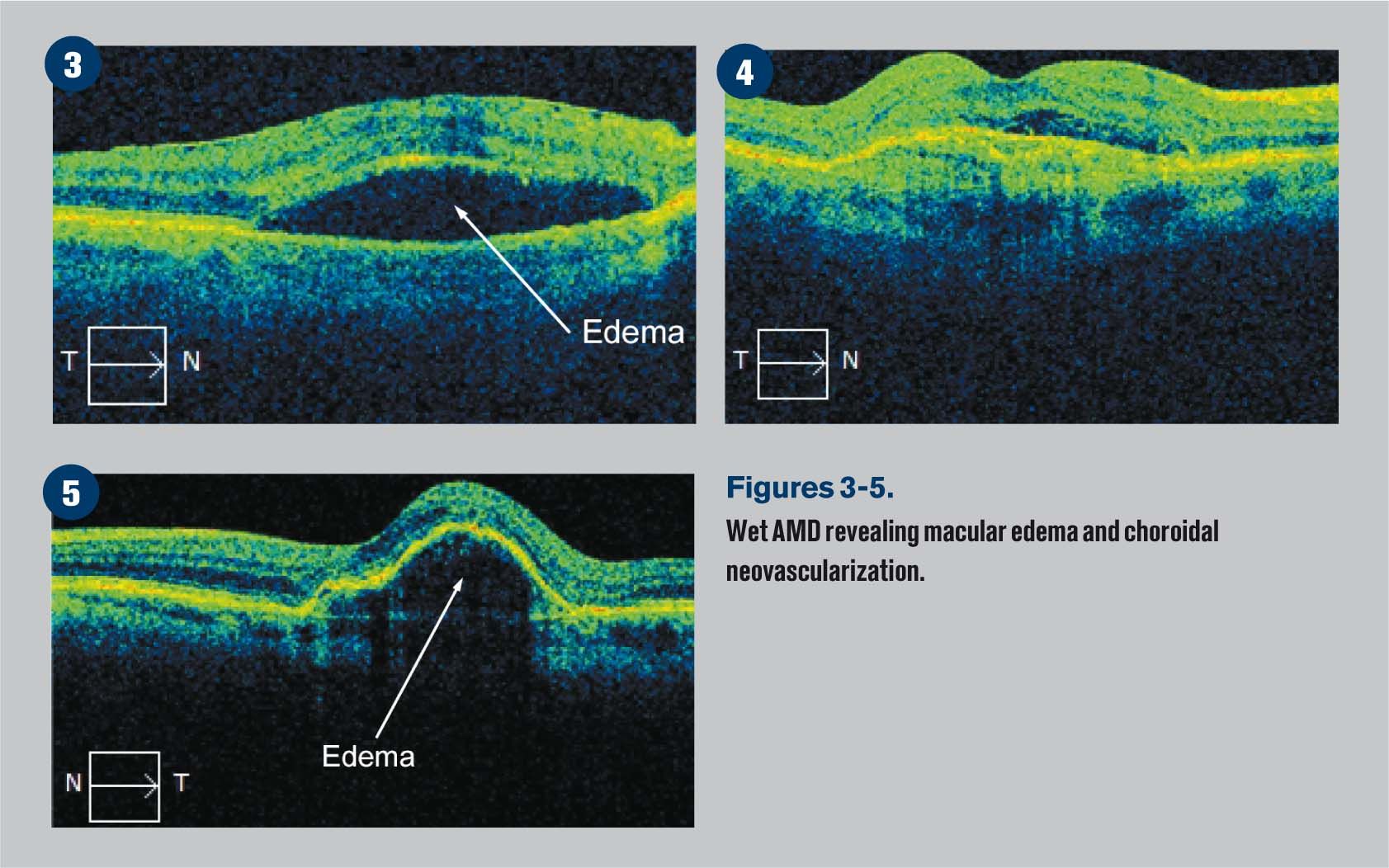
Cystoid macular edema (CME) is the accumulation of fluid in the outer plexiform layer secondary to abnormal perifoveal retinal capillary permeability. The edema is termed "cystoid" because it appears cystic; however, lacking an epithelial coating, it is not truly cystic.
There are various etiologies for CME. The most common are:3
• Diabetes
• Epinepherine
• Pars planitis
• Retinitis pigmentosa
• Irvine-Gass syndrome
• Venous occlusion
• E2-prostaglandin analogues
• Retinal detachment
• Sarcoid
• Nicotinic acid/Niacin
Related: Detect, treat pre-symptomatic age-related macular degeneration
Patients will present with decreased vision and metamorphopsia. Clinical findings include decreased foveal light reflex, foveal thickening, with or without small intraretinal cysts. In severe cases there can be optic nerve swelling and vitreous cells.

A foveal cyst, or a lamellar macular hole, may develop, causing permanent vision loss. Pseudophakic cystoid macular edema, also known as Irvine-Gass syndrome, is CME occurring after cataract surgery with peak incidence six to 10 weeks post operatively. This may occur in both uncomplicated and complicated cataract surgery.
Figure 5 reveals cystic areas of edema in this classic case of CME.
Epiretinal membrane
An epiretinal membrane is a fibroglial membrane proliferation on the pre-macular area causing a distortion of normal macular architecture.4 Early presentation will show a glittery light reflex in the macula and vision is often normal with no visual symptoms.
A mild membrane will present with increased cellophane appearance, wrinkling, and distortion of retinal capillaries along with mild metamorphopsia. Vision is often 20/40 or better. A macular pucker will show significant distortion of the retinal vessels and wrinkling with edema and/or thickening of the internal limiting membrane.4
Related: Stem cell trial aims to cure AMD
Epiretinal membranes are often seen in patients with no history of ocular problems. They are often idiopathic but can be associated with diabetes, trauma, or a history of a torn or detached retina. A natural change in the vitreous causes glial cell proliferation between the internal limiting membrane and the posterior hyaloid membrane of the vitreous. It settles on the macula and forms a membrane.5
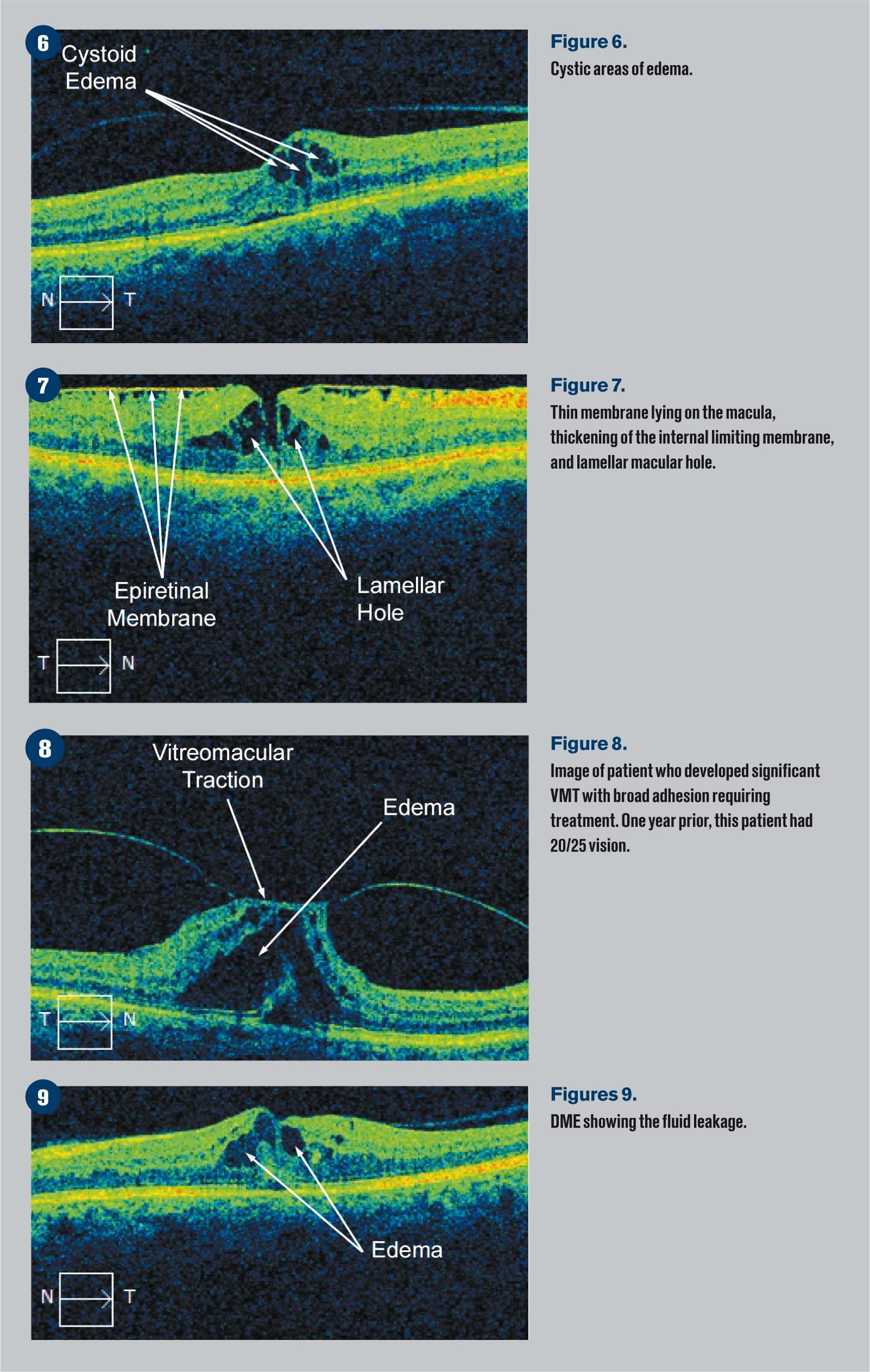
Figure 6 shows a thin membrane lying on the macula as well as some thickening of the internal limiting membrane and a lamellar macular hole.
Vitreomacular traction (VMT)
With age, the vitreous slowly liquefies. This is usually seen in people over age 50 as well as high myopes and aphakes. The vitreous interface will weaken, and the vitreous detaches. This is called a posterior vitreous detachment (PVD).
About 68 percent of people over age 65 have PVDs. PVDs can occur in all ethnicities, and 60 to 70 percent of PVDs and VMTs occur in women. Most non-traumatic VMTs occur in individuals age 60 to 80.5 As this process occurs, many individuals will have incomplete dehiscence, causing vitreomacular adhesions.6
Related: Current views on genetic testing for AMD
Vitreomacular adhesions can progress to vitreomacular traction, which can cause debilitating visual symptoms. Left untreated, vitreomacular traction can cause an increased risk of a full thickness macular hole.7 The course of VMT is variable. It can progress abruptly or slowly and can even resolve spontaneously.

Figure 7 demonstrates a patient who developed significant VMT with a rather broad adhesion requiring treatment. One year prior this patient had 20/25 vision.
Macular hole
A macular hole is a dehiscence of retinal tissue in the macula in the form of a round hole. It may be due to age (idiopathic), trauma, vitreomacular traction, or CME with rupture of a cyst. In an idiopathic macular hole, you will see contraction of the adherent prefoveolar vitreous cortex causing an early foveal detachment.
Lamellar holes involve the inner layers of the macular where the photoreceptor layer is intact.8 Full-thickness holes involve all layers down to the RPE.
A patient with an impending hole will often have normal vision but experience metamorphopsia. Full-thickness holes can cause vision ranging from 20/50 to 20/800.
There are four stages of macular holes according to the Gass Classification system:9
• Stage 1a: Central yellow spot indicating a foveal detachment, loss of foveal reflex and depression. Vitreous attached at fovea
• Stage 1b: Yellow ring with bridging interface, loss of foveal reflex and depression. Vitreous attached at fovea
• Stage 2: Round, oval, crescent, or horseshoe-shaped retinal defect within yellow ring. A pre-foveal opacity or operculum may or may not be present. There is less than 400 micrometers or retinal dehiscence present
• Stage 3: Central round full thickness retinal defect greater than 400 micrometers. Pre-macular vitreous is still attached. No Weiss ring present because vitreous still attached to optic nerve head. Positive Watzke’s sign where a narrow slit beam is presented on the fovea, and patient reports a broken line of light
• Stage 4: Same as stage 3 but with a PVD and Weiss ring present
Figure 8 shows a macular hole appearance, and Figure 9 shows a full thickness macular hole.
Related: Determining the value in genetic testing for AMD
Diabetic macular edema
Diabetic macular edema (DME) is caused by leaking macular capillaries. With DME, breakdown of the inner blood retinal barrier allows extracellular fluid to accumulate in the macula.10 DME is the most common cause of visual loss in both proliferative, and non-proliferative diabetic retinopathy.3
Findings include hard exudates, micro-aneurysms, and focal or diffuse macular edema or thickening, which can be cystic.
Remember the following guidelines as set forth by the Early Treatment for Diabetic Retinopathy Study (ETDRS) regarding referral recommendations:4
• Retinal thickening or edema within 500 micrometers (one-third of a disc diameter) of the center of the fovea
• Hard exudates within 500 micrometers of the center of the macula, associated with retinal thickening
• Retinal thickening greater than one disc area in size, part of which is within one disc diameter of the center of the fovea
I follow these guidelines when referring based on my 90-D evaluation and OCT findings.
Figure 9 is a classic example of DME showing the fluid leakage.
Central serous retinopathy
Central serous retinopathy (CSR) is a serous detachment of the neurosensory retina in the macula due to an alteration of the RPE and choroidal circulation. It is typically seen in males with type-A personality between ages 25 to 50 years with central blurring vision and micropsia.
Serous detachments in these individuals is thought to be due to elevated plasma catecholamine levels. Clinical findings include a yellow, blister-like elevation of the sensory retina in the macula.
CSR has also been associated with cortisol and corticosteroids. Persons with CSR have higher levels of cortisol, a hormone secreted by the adrenal cortex that allows the body to deal with stress, which may explain the CSR-stress association. Studies show that corticosteroids can trigger, exacerbate, and cause recurrences of CSR.
Related: New devices help fine tune age-related macular degeneration management
CSR has been shown to develop in patients using inhaled or intranasal steroids. Glucocorticoids are believed to cause CSR by increasing catecholamine release, which may lead to pump dysfunction at the RPE or increase permeability of the choroidal vasculature.
The dysfunction of the RPE pump or the vascular permeability is thought to cause CSR.11
The prognosis for CSR is generally excellent. Initially, visual acuity may be as poor as 20/200 in the affected eye. However, over 90 percent of patients regain 20/30 vision or better within 6 months.11
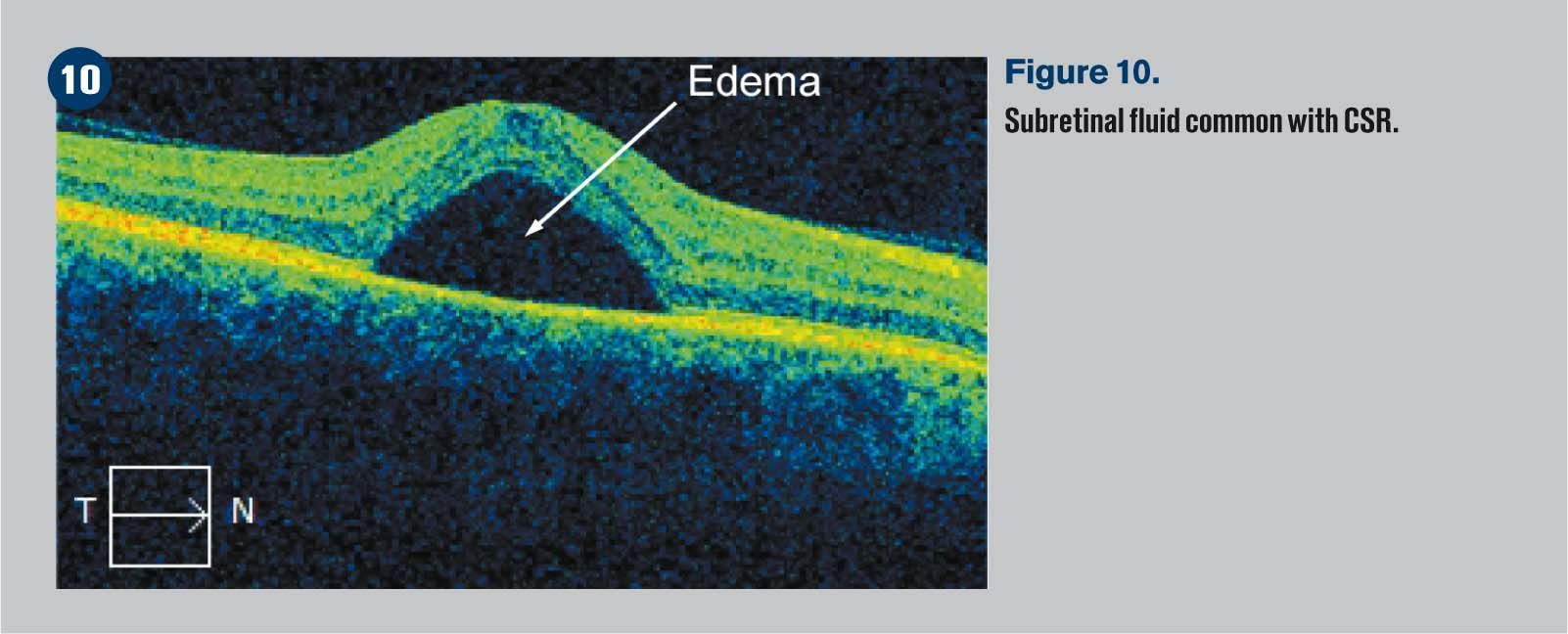
Figure 10 shows subretinal fluid common with CSR.
Chloroquine retinopathy
Retinal toxicity associated with hydroxychloroquine use is relatively rare, estimated at one percent after five years and rising with continued therapy. In cases of severe retinopathy, described as a bull’s eye maculopathy, no known treatment exists and may even progress after cessation of the drug.12
Hydroxychloroquine (Plaquenil, Sanofi) is an antimalarial drug that has gained widespread use in treating various autoimmune diseases, including systemic lupus erythematosus and rheumatoid arthritis. It is estimated that more than 150,000 patients are on long-term therapy in America alone.12
Related: Telescopic contact lens could help AMD patients
Published case reports of chloroquine retinopathy rarely include details of daily dosage, but 30 reports in which this information was available included 78 patients who developed impaired visual acuity, of which 13 had received daily doses of 250 mg or less.
Of the 11 cases of ocular toxicity attributed to chloroquine that were reported to the Committee on Safety of Medicines, only two cases developed impaired vision on 250 mg daily.13
A postal questionnaire among 41 rheumatology and 33 ophthalmology centers showed two patients who developed impaired vision as a result of treatment with chloroquine in a daily dose not exceeding 250 mg. Serious visual impairment related to chloroquine rarely occurs if the daily dose does not exceed 250 mg.13
Related: Addressing AREDS2 controversies
Visual symptoms include difficulty adjusting to darkness, glare, paracentral scotomas, decreased vision, and abnormal color vision. Signs include loss of foveal reflex, bull’s eye maculopathy, and whorl-like corneal changes.
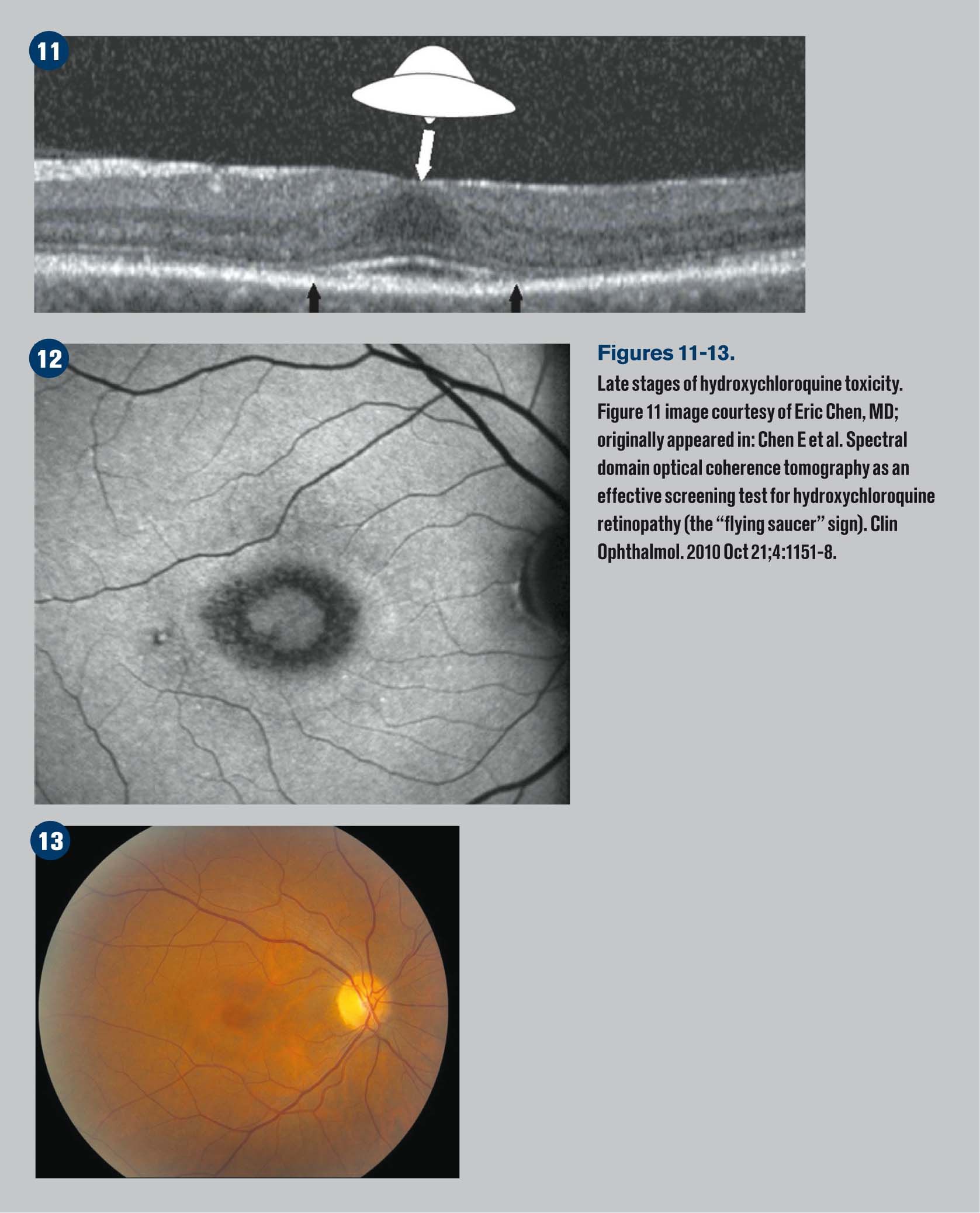
OCT findings can show loss of the external limiting membrane, disruption of the outer ellipsoid zone (IS/OS junctions), perifoveal thinning of the outer nuclear layer, RPE damage, and “flying saucer sign.”
The flying saucer sign gives an ovoid appearance on the OCT that is created by the intact central foveal outer retinal layers surrounded by perifoveal loss of the photoreceptor ellipsoid band and outer nuclear layer atrophy.
The intact central foveal outer retinal layer, referred to as foveal resistance, explains intact central visual acuity seen in advanced toxicity cases.12 It is important to relay these findings to the patient's endocrinologist and primary care physician so the medication can be stopped.
Related: New device aids early AMD diagnosis
Figures 11-13 demonstrate late stages of hydroxychloroquine toxicity.
As primary care optometrists, getting comfortable with these conditions and the interpretation of the OCT findings allows you to better inform patients regarding the condition as well as the severity of their case and the recommended treatment options.
References:
1. Filho MA, Witkin AJ. Outer retinal layers as predictors of vision loss. Rev Ophthalmol. Available at: https://www.reviewofophthalmology.com/article/outer-retinal-layers-as-predictors-of-vision-loss. Accessed 11/4/16.
2. Amin HI, McDonald R, Johnson RN, Ai E, Schatz, H. Chapter 23: Acquired Macular Disease. Duane’s Ophthalmology. Volume 3. Available at: http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v3/v3c023.html#cys. Accessed 11/8/16.
http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v3/v3c023.html
3. Amin HI, McDonald R, Johnson RN, Ai E, Schatz, H. Chapter 23: Cystoid Macular Edema. Duane’s Ophthalmology. Volume 3. Available at: http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v3/v3c023.html#cys. Accessed 11/8/16.
4. Mandava, Suresh, Tara Sweeney, and David R. Guyer. Color Atlas of Ophthalmology: The Manhattan Eye, Ear & Throat Hospital Pocket Guide. New York: Thieme, 1999. 265, 325.
5. Joyce K, Gurwood AS. A look at VMT Syndrome. Rev Optom. 2011 Oct 15. Available at: https://www.reviewofoptometry.com/article/a-look-at-vmt-syndrome. Accessed 11/8/16.
6. Heier JS. Current and potential uses of ocriplasmin. Rev Ophthalmol. Available at: https://www.reviewofophthalmology.com/article/current-and-potential-uses-of-ocriplasmin. Accessed 11/4/16.
7. Kaiser PK. Ocriplasmin for VMT: A review of safety data. Rev Ophthalmol. Available at: http://www.reviewofophthalmology.com/article/ocriplasmin-for-vmt-a-review-of-safety-data. Accessed 11/4/16.
8. Xirou T, Kidess A, Kourentis C, Xirou V, Feretis E, Kabanarou SA. Lamellar macular hole formation following vitrectomy for rhegmatogenous retinal detachment repair. Clin Ophthalmol. 2012;6:571-4.
9. Lowenstein JI. Macular Hole. Digital J Ophthalmology. 2003 Jan 9. Available at: http://www.djo.harvard.edu/site.php?url=/patients/pi/532. Accessed 11/8/16.
10. Ciulla TA. Corticosteroids for diabetic macular edema. Rev Ophthalmol. Available at: https://www.reviewofophthalmology.com/article/corticosteroids-for-diabetic-macular-edema-2015. Accessed 11/4/16.
11. Amin HI, McDonald R, Johnson RN, Ai E, Schatz, H. Chapter 23: Central Serous Retinopathy. Duane’s Ophthalmology. Volume 3. Available at: http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v3/v3c023.html#cys. Accessed 11/8/16.
12. Rahimy E, Vander J. Multimodal imaging in plaquenil toxicity. Rev Ophthalmol. Available at: https://www.reviewofophthalmology.com/article/multimodal-imaging-in-plaquenil-toxicity. Accessed 11/4/16.
13. Marks JS. Chloroquine retinopathy: is there a safe daily dose? Ann Rheum Dis. 1982 Feb;41(1):52-8.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.