Is complement therapy the path forward for geographic atrophy?
Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider.
Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider. (Adobe Stock/Jevgenij)
Geographic atrophy (GA), an advanced form of age-related macular degeneration (AMD), is a progressive eye disease characterized by atrophic retinal lesions that develop because of loss of photoreceptors, retinal pigment epithelium (RPE), and the underlying choriocapillaris, resulting in irreversible vision loss.1
AMD is a complex and multifactorial disease, and its underlying pathophysiology has not been elucidated. However, there is increasing evidence that the dysregulation of the complement system, an important component of innate immunity, is implicated in AMD and, therefore, GA pathogenesis.2-5
Several components of the complement system have been considered attractive therapeutic targets for GA, with many investigational therapies in clinical development.3,6,7 Notably, in February 2023, the FDA approved pegcetacoplan injection (Syfovre) as the first treatment for GA, and another investigational therapy, avacincaptad pegol, is under FDA review.8,9 Both are complement-targeting therapies, which further underscores the role of complement in GA pathogenesis.
The complement system identifies and eliminates pathogens and dying cells.2,3,6,7,10-13 In brief, 3 pathways can activate the complement system—classical, lectin, and alternative—and their activation results in similar functions of opsonization, inflammation, and cell lysis.
Each pathway converges with the cleavage of complement C3 into C3a and C3b by C3 convertase, and the formation of C5 convertase, which cascades down to C5 convertase, cleaving C5 to C5a and C5b, initiating the terminal cascade of the complement system. C5b leads to the formation of a membrane attack complex (MAC; C5b-9), which creates pores in the cell membrane of targeted cells and leads to cell lysis and death.2,6,10-12,14,15
Furthermore, C3b not only aids in the formation of the C5 convertase, but it also facilitates phagocytosis and the transport of opsonized cells to the spleen or liver by opsonizing pathogens.16,17 C3a and C5a are anaphylatoxins. C5a has strong proinflammatory effects and may play a role in priming RPE cells for inflammasome activation, leading to pyroptosis, whereas C3a initiates proinflammatory or anti-inflammatory responses in a context-dependent manner.10,12,18-22
There are also several complement regulatory proteins that tightly regulate the complement system.6,10,11 The complement system has been implicated in GA pathogenesis based on decades of preclinical, histological, and genetic research and, more recently, clinical trials demonstrating a benefit of complement inhibition in slowing progression of disease in patients with GA.2,23-27
Numerous genetic studies, including genomewide association studies, have identified various complement genes linked to AMD, which include polymorphisms in C3, C2/CFB, CFI, and CFH.13,27-34 Immunochemistry findings have demonstrated that aging and AMD are associated with increased MAC accumulation in the choriocapillaris, the presence of complement components and MAC in drusen, and elevated complement components at the interface of Bruch membrane and the choroid in eyes with advanced AMD vs control eyes.30,35-38
Based on the aforementioned scientific evidence, it is hypothesized that overactivation of the complement system and reduced expression of complement regulators, which can be compounded by genetic and environmental risk factors, may result in inflammation because of the accumulation of complement activation products and MAC in drusen.
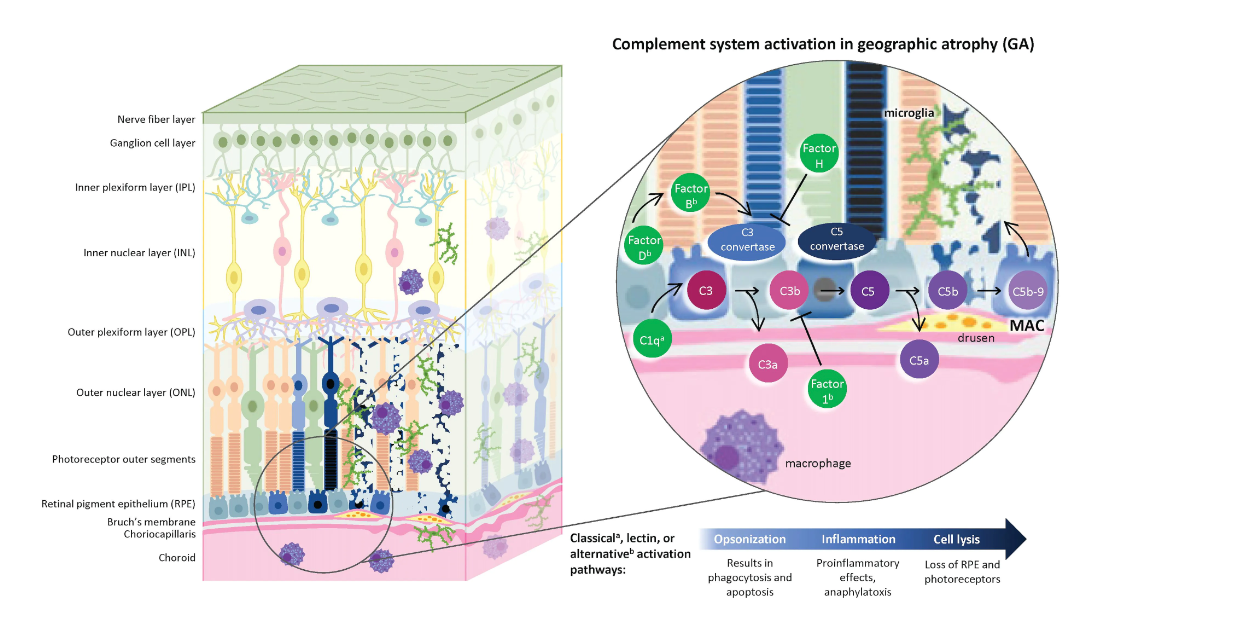
Figure The Complement Pathway and Geographic Atrophy6,11,13,15,40-43
GA is a progressive eye disease characterized by the loss of photoreceptors, RPE, and the choriocapillaris, resulting in vision loss. Overactivation of the complement system, which normally eliminates pathogens or dying cells, has been implicated in the inflammatory processes driving GA. Three pathways can activate the system (classical, lectin, or alternative), all culminating in similar pathways of opsonization, inflammation, and cell lysis. The pathways converge with the cleavage of complement C3 into C3a and C3b by C3 convertase. C5 convertase is then formed, which cleaves C5 to C5a and C5b. During these stages of complement activation, C3b promotes phagocytosis and opsonization, while C3a and C5a act as anaphylatoxins and promote inflammation. The terminal cascade is then initiated by C5b with the development of the MAC (c5b-9), which, in turn, leads to the formation of openings in the cell membrane of targeted cells, followed by cell lysis
and cell death.
Under normal circumstances, there are several complement regulatory proteins that tightly regulate the complement system. As AMD progresses, however, the signs of overactive complement activation gather, with affected tissues exhibiting increased accumulation of MAC in the choriocapillaris and drusen, and increased complement components within drusen and the interface of Bruch membrane and the choroid. Collectively, this process damages Bruch membrane and can lead to the loss of RPE and photoreceptors, ultimately contributing to GA. As a result, multiple points along the complement activation pathway have emerged as therapeutic targets, including inhibitors of C3 and C5, and investigational therapies targeting factor B, factor 1, C1q, factor D, and factor H.
Figure courtesy of Jaclyn L. Kovach, MD
This, in turn, results in damage to Bruch membrane and can lead to the loss of RPE and photoreceptors, which ultimately contribute to the development of GA (Figure).6,11,13,15,39-43 A focus of continued discussion is which point in the complement cascade should be targeted as a therapeutic intervention to reduce GA lesion growth and slow disease progression, possibly preserving vision.
The 2 most developed therapies for GA are C3 and C5 inhibitors. Notably, pegcetacoplan injection, which inhibits C3 of the complement cascade, has drawn attention as the first FDA-approved therapy for GA.9
C3 is an attractive therapeutic target because all 3 complement pathways converge at C3 and inhibiting C3 will reduce the generation of anaphylatoxins (C3a, C5a) and MAC, which may preserve RPE cells and photoreceptors.13,26 It is hypothesized that an accumulation of C3 fragments in the RPE, photoreceptors, and/or capillary endothelial cell surfaces because of oxidative stress-mediated reduction in endocytosis promotes phagocytosis, which can be prevented with C3 inhibition and allow for cell survival.26
C5 inhibition is also a favorable therapeutic target because it may slow GA progression with more direct inhibition of MAC by preventing the production of terminal, active C5 cleavage products (C5a and C5b), regardless of the initiating pathway.
Depending on the cell type and cell defense mechanisms, MAC can cause cell lysis, cause cell death by apoptosis, or induce inflammatory responses that can lead to tissue damage if MAC is sublytic.2,12,14,25,45-47 Further, C5 inhibition may preserve potentially beneficial upstream functions of the complement cascade, such as the neuroprotective and anti-inflammatory activity of C3 and C3a.19,48-50
Importantly, both C3 and C5 inhibition prevent the terminal complement pathway and have significantly slowed GA lesion growth compared with sham in patients with GA in phase 3 clinical trials, demonstrating clinical benefit in modifying the disease’s trajectory.23-26 It should be noted that several other investigational therapies targeting different areas of the complement system are in phase 2 development, including factor B, factor 1, C1q, factor D, and factor H.3,6,7
Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider. The complement system is important for maintaining retinal homeostasis during the aging process,50,51 and it may be overactivated in GA. Therefore, long-term studies are needed to further evaluate the impact of complement inhibition. Some of the complement therapies have shown an increase in macular neovascularization, intraocular inflammation, increased IOP, endophthalmitis, and retinal detachments with complement inhibition.
However, incidence rates in clinical trials were low,25,26,52-54 and these conditions can be managed. Lastly, there have been no improvements in vision based on prespecified analyses with therapies inhibiting the complement system.25,26 However, improvements in best-corrected visual acuity are not expected with these therapies because the goal of complement inhibition is to slow disease progression; tissue loss will not be regained. Furthermore, exploratory post hoc analyses have demonstrated a reduction in the risk of vision loss, showing the potential of these therapies to prevent disease progression.55
Conclusion
The first FDA approval of a GA therapy will change GA management by eye care professionals, which includes ensuring patients are referred to retina specialists to provide them with the treatment needed. With more therapies under FDA review and investigation, it brings into question which patients will benefit most from complement inhibition and what the optimal treatment window is. This warrants further clinical investigation, guidelines, and expert consensus for eye care professionals.
According to one study, levels of complement activation (systemic C3d/C3 ratio) were shown to increase with each progressive stage of AMD, particularly in patients who had a genetic susceptibility to specific complement genes. Patients with intermediate AMD and central GA had the highest levels of complement activation vs patients with other stages of AMD.56
In summary, overactivation of the complement system has been implicated in the pathogenesis of GA, which leads to excessive inflammation and cell death and can lead to the damage or loss of RPE, photoreceptors, and choriocapillaris and contribute to the loss of vision. The advent of approved complement pathway-targeted therapies for GA provides patients and retinal specialists with an opportunity to manage GA.
Jaclyn L. Kovach, MD
e: email@gmail.com
Jaclyn L. Kovach, MD, is a professor of clinical ophthalmology in the Department of Ophthalmology and Visual Sciences at the University of Miami in Florida.
References
Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390. doi:10.1016/j.ophtha.2017.08.038
Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina.2017;37(5):819-835. doi:10.1097/IAE.0000000000001392
Desai D, Dugel PU. Complement cascade inhibition in geographic atrophy: a review. Eye (Lond). 2022;36(2):294-302. doi:10.1038/s41433-021-01765-x
Keenan TDL. Local complement inhibition for geographic atrophy in age-related macular degeneration: prospects, challenges, and unanswered questions. Ophthalmol Sci.2021;1(4):100057. doi:10.1016/j.xops.2021.100057
Park YG, Park YS, Kim IB. Complement system and potential therapeutics in age-related macular degeneration. Int J Mol Sci.2021;22(13):6851. doi:10.3390/ijms22136851
Armento A, Ueffing M, Clark SJ. The complement system in age-related macular degeneration. Cell Mol Life Sci.2021;78(10):4487-4505. doi:10.1007/s00018-021-03796-9
Patel PN, Patel PA, Land MR, Bakerkhatib-Taha I, Ahmed H, Sheth V. Targeting the complement cascade for treatment of dry age-related macular degeneration. Biomedicines. 2022;10(8):1884. doi:10.3390/biomedicines10081884
Iveric Bio announces completion of rolling NDA submission to FDA for avacincaptad pegol for the treatment of geographic atrophy. News release. IVERIC Bio. December 20, 2022. Accessed May 25, 2023. https://www.businesswire.com/news/home/20221219005815/en/Iveric-Bio-Announces-Completion-of-Rolling-NDA-Submission-to-FDA-for-Avacincaptad-Pegol-for-the-Treatment-of-Geographic-Atrophy
FDA approves SYFOVRE (pegcetacoplan injection) as the first and only treatment for geographic atrophy (GA), a leading cause of blindness. News release. Apellis. February 17, 2023. Accessed May 25, 2023. https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only
Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34(22):2735-2757. doi:10.15252/embj.201591881
Chirco KR, Potempa LA. C-reactive protein as a mediator of complement activation and inflammatory signaling in age-related macular degeneration. Front Immunol.2018;9:539. doi:10.3389/fimmu.2018.00539
Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: from rare diseases to pandemics. Pharmacol Rev. 2021;73(2):792-827. doi:10.1124/pharmrev.120.000072
van Lookeren Campagne M, Strauss EC, Yaspan BL. Age-related macular degeneration: complement in action. Immunobiology.2016;221(6):733-739. doi:10.1016/j.imbio.2015.11.007
Xie CB, Jane-Wit D, Pober JS. Complement membrane attack complex: new roles, mechanisms of action, and therapeutic targets. Am J Pathol.2020;190(6):1138-1150. doi:10.1016/j.ajpath.2020.02.006
Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol.2016;787:94-104. doi:10.1016/j.ejphar.2016.03.001
Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res.2010;20(1):34-50. doi:10.1038/cr.2009.139
Ricklin D, Reis ES, Mastellos DC, et al. Complement component C3 - the “Swiss Army knife” of innate immunity and host defense. Immunol Rev.2016;274(1):33-58. doi:10.1111/imr.12500
Brandstetter C, Holz FG, Krohne TU. Complement component C5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin-mediated photooxidative damage. J Biol Chem. 2015;290(52):31189-31198.doi:10.1074/jbc.M115.671180
Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol.2015;194(8):3542-3548. doi:10.4049/jimmunol.1403068
Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol.2005;23:821-852. doi:10.1146/annurev.immunol.23.021704.115835
Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: key concepts and lingering questions. Prog Retin Eye Res.2021;83:100936.doi:10.1016/j.preteyeres.2020.100936
Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a). Int J Biochem Cell Biol.2009;41(11):2114-2117. doi:10.1016/j.biocel.2009.04.005
Steinle N, Boyer D, Heier J, et al. Efficacy of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials. Presented at: American Society of Retina Specialists Annual Scientific Meeting; October 8-12, 2021; San Antonio, TX.
Khanani AM, Patel SS, Staurenghi G, et al. The efficacy of avacincaptad pegol in geographic atrophy: GATHER1 and GATHER2 results. Presented at: The Retina Society Annual Scientific Meeting; November 2-5, 2022; Pasadena, CA.
Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology.2021;128(4):576-586. doi:10.1016/j.ophtha.2020.08.027
Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology.2020;127(2):186-195. doi:10.1016/j.ophtha.2019.07.011
Toomey CB, Johnson LV, Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res.2018;62:38-57. doi:10.1016/j.preteyeres.2017.09.001
Edwards AO, Ritter R 3rd, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science.2005;308(5720):421-424. doi:10.1126/science.1110189
Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet.2013;45(4):433-439. doi:10.1038/ng.2578
Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227-7232. doi:10.1073/pnas.0501536102
Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419-421. doi:10.1126/science.1110359
Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385-389. doi:10.1126/science.1109557
Li M, Atmaca-Sonmez P, Othman M, et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38(9):1049-1054. doi:10.1038/ng1871
Sofat R, Casas JP, Webster AR, et al. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int J Epidemiol. 2012;41(1):250-262. doi:10.1093/ije/dyr204
Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest Ophthalmol Vis Sci.2008;49(2):743-750. doi:10.1167/iovs.07-1072
Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res.2000;70(4):441-449. doi:10.1006/exer.1999.0798
Loyet KM, Deforge LE, Katschke KJ Jr, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci.2012;53(10):6628-6637. doi:10.1167/iovs.12-9587
Mullins RF, Schoo DP, Sohn EH, et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184(11):3142-3153. doi:10.1016/j.ajpath.2014.07.017
Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1-29. doi:10.1016/j.preteyeres.2014.11.005
Jha P, Bora PS, Bora NS. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol.2007;44(16):3901-3908. doi:10.1016/j.molimm.2007.06.145
Grigoryan EN. Self-organization of the retina during eye development, retinal regeneration in vivo, and in retinal 3D organoids in vitro. Biomedicines. 2022;10(6). doi:10.3390/biomedicines10061458
Yednock T, Fong DS, Lad EM. C1q and the classical complement cascade in geographic atrophy secondary to age-related macular degeneration. Int J Retina Vitreous. 2022;8(1):79. doi:10.1186/s40942-022-00431-y
Khandhadia S, Cipriani V, Yates JRW, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217(2):127-146. doi:10.1016/j.imbio.2011.07.019
Tan LX, Germer CJ, La Cunza N, Lakkaraju A. Complement activation, lipid metabolism, and mitochondrial injury: converging pathways in age-related macular degeneration. Redox Biol.2020;37:101781. doi:10.1016/j.redox.2020.101781
Tan LX, Toops KA, Lakkaraju A. Protective responses to sublytic complement in the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2016;113(31):8789-8794. doi:10.1073/pnas.1523061113
Nauta AJ, Daha MR, Tijsma O, van de Water B, Tedesco F, Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol.2002;32(3):783-792. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q
Shinjyo N, de Pablo Y, Pekny M, Pekna M. Complement peptide C3a promotes astrocyte survival in response to ischemic stress. Mol Neurobiol.2016;53(5):3076-3087. doi:10.1007/s12035-015-9204-4
Silverman SM, Ma W, Wang X, Zhao L ,Wong WT. C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J Exp Med. 2019;216(8):1925-1943. doi:10.1084/jem.20190009
van Beek J, Nicole O, Ali C, et al. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport.2001;12(2):289-293. doi:10.1097/00001756-200102120-00022
Mukai R, Okunuki Y, Husain D, Kim CB, Lambris JD, Connor KM. The complement system is critical in maintaining retinal integrity during aging. Front Aging Neurosci.2018;10:15. doi:10.3389/fnagi.2018.00015
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol.2010;11(9):785-797. doi:10.1038/ni.1923
Boyer D, Steinle N, Wykoff C, et al. Safety of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials. Presented at: American Society of Retina Specialists Annual Scientific Meeting; October 8-12, 2021; San Antonio, TX.
Kaiser PK, Khanani AM, Eichenbaum DA, et al. Safety of intravitreal avacincaptad pegol in geographic atrophy: GATHER1 and GATHER2 results. Presented at: The Retina Society Annual Scientific Meeting; November 2-5, 2022; Pasadena, CA.
SYFOVRE. Prescribing information. Apellis Pharmaceuticals Inc; 2023.
Iveric Bio announces vision loss reduction data in geographic atrophy from avacincaptad pegol GATHER trials. News release. Iveric Bio. March 1, 2023. Accessed April 5, 2023. https://investors.ivericbio.com/news-releases/news-release-details/iveric-bio-announces-vision-loss-reduction-data-geographic
Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40(2):140-170. doi:10.1111/opo.12675
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.