J&J Vision receives FDA clearance for astigmatism management software
Catalys cOS 6.0 allows for more accurate laser alignment
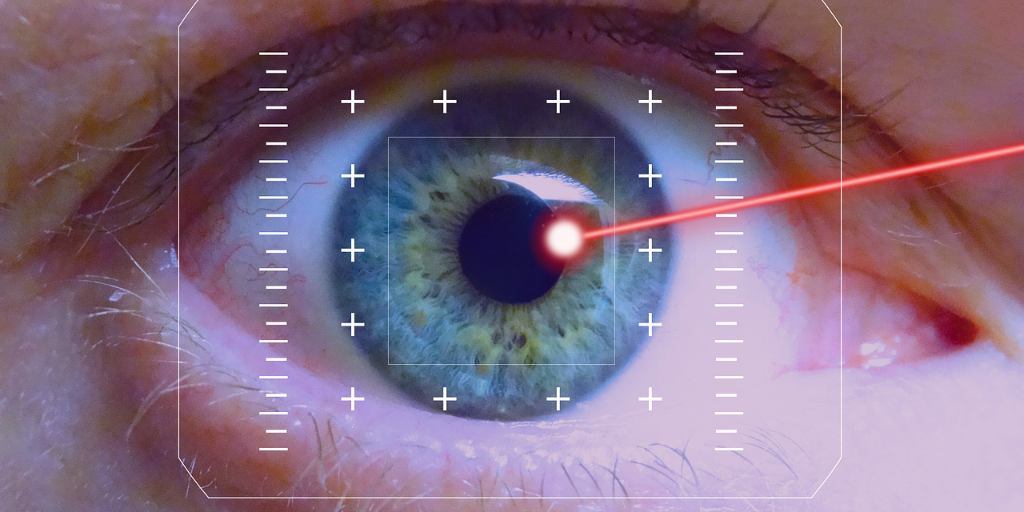
Johnson & Johnson Vision’s new astigmatism management software, Catalys cOS 6.0, received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The software package is used in conjunction with its laser cataract surgery system to enable more accurate laser alignment.
It automatically compensates for cyclorotation, or torsional movement of the eye, according to the company.
The astigmatism software offers new features that streamline workflow by speeding up surgical processes and improving accuracy for surgeons using the Catalys Precision Laser system. According to J&J Vision, it does this by:
- Minimizing manual transcription errors and data entry verification through direct input of preoperative keratometry and steep axis values data
- Reducing calculation time by including calculations of arcuate incision parameters using built-in nomogram and automatic input of arcuate incision parameters in the treatment planning screen
- Reducing the need for intraoperative aberrometry for toric IOL alignment with radial laser marks placed within a mean accuracy of 0.6 degrees.
- Adding iris registration for automatic cyclorotation compensation
Catalys cOS 6.0 will be available in the fourth quarter of 2020.
Read more: AOA elects '20-'21 officer slate via virtual ballot
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.