Loteprednol delivers pain relief
A post hoc analysis of data collected in two published, pivotal clinical trials demonstrates that in addition to its efficacy for treating inflammation following ocular surgery, loteprednol etabonate ophthalmic suspension 0.5% provides statistically significant relief of postoperative pain and other symptoms.
Rochester, NY-A post hoc analysis of data collected in two published, pivotal clinical trials [Ophthalmology. 1998;105:1780-1786 and J Cataract Refract Surg. 1998;24:1480-1489] demonstrates that in addition to its efficacy for treating inflammation following ocular surgery, loteprednol etabonate ophthalmic suspension 0.5% (Lotemax, Bausch + Lomb) provides statistically significant relief of postoperative pain and other symptoms, said Timothy L. Comstock, OD, MS.
"The new drug application for [loteprednol etabonate] was submitted in the late 1990s, and, at that time, the indication sought for postoperative use was specifically for treating anterior chamber inflammation," Dr. Comstock said. "However, because inflammation is accompanied by pain, it's no surprise that by providing effective control of inflammation, loteprednol etabonate also would offer relief from pain."
Anterior chamber cell, flare reaction
A total of 430 enrolled patients were randomly assigned 1:1 to use loteprednol etabonate or vehicle in the operated eye four times daily for up to 14 days.
Follow-up visits were conducted on postoperative days 3, 8, 15, and 17, and in addition to the evaluations of anterior chamber cell and flare reaction, data were collected on the presence and severity of a variety of ocular inflammatory signs and symptoms, including pain. Severity ratings were made using a scale of 0 (absent) to 3 (severe).
The final analysis
At the final visit in both studies, the loteprednol etabonate-treated groups had greater improvements from baseline mean pain scores compared with the vehicle-treated controls as well as significantly higher rates of pain resolution (p = 0.003 and p = 0.018, respectively).
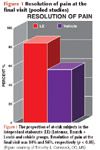
Figure 1 shows that an analysis based on pooled data from the two trials showed that among patients experiencing pain after surgery, resolution was achieved at the final visit in 84% of loteprednol etabonate-treated patients and 56% of vehicle-treated controls (p <0.05).
Other symptoms assessed included photophobia, itching, tearing, dryness, discharge, and discomfort. In both studies, based on final visit data for at-risk eyes having a baseline complaint, resolution rates were significantly higher in the loteprednol etabonate groups compared with the controls in analyses of photophobia (65% versus 41%; 67% versus 44%), tearing (84% versus 60%; 80% versus 55%), and discomfort (77% versus 34%; 82% versus 53%).
"The results on discomfort ratings are consistent with other clinical trials that have shown good tolerability for [loteprednol etabonate]," Dr. Comstock said.
"[Loteprednol etabonate] is a very comfortable formulation," he said in conclusion. "This feature may be attributed in part to the fact that its vehicle contains glycerin and povidone, two ingredients that are known demulcents and used for improving the comfort of topical ophthalmic preparations."
FYI
Timothy L. Comstock, OD, MS
E-mail: tcomstock@bausch.com
Dr. Comstock is an employee of Bausch + Lomb, but has no other direct financial interest in the subject matter.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.