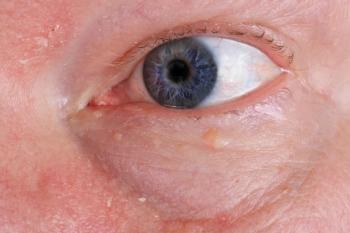
MS medication may be associated with macula edema
Gilenya (Fingolimod, Novartis), a sphingosine-1-phosphate receptor modulator, became the first FDA-approved oral agent for the treatment of relapsing forms of multiple sclerosis (MS) in September 2010.1 Recently, it has been associated with macular edema, referred to as FAME, or Fingolimod-associated macular edema.
Gilenya (Fingolimod, Novartis), a sphingosine-1-phosphate receptor modulator, became the first FDA-approved oral agent for the treatment of relapsing forms of multiple sclerosis (MS) in September 2010.1 Recently, it has been associated with macular edema, referred to as FAME, or Fingolimod-associated macular edema.
TRANSFORMS and FREEDOMS
Two recent Phase III clinical studies (TRANSFORMS2 [Trial Assessing Injectable interferon vs. FTY720 Oral in RRMS] and FREEDOMS3 [FTY720 Research Evaluation Effects of Daily Oral Therapy in MS]) demonstrated significant reduction in the annualized relapse rate in patients with relapsing-remitting MS, compared to once-weekly interferon B-1a and placebo. In the FREEDOMS trial, 1,272 patients were randomized to treatment with 0.5mg/day fingolimod, 1.25mg/day fingolimod, or placebo for 24 months.2 The annualized relapse percentage was 0.18, 0.16, and 0.40, respectively. In the TRANSFORMS trial, 1,292 patients were randomized to treatment with 0.5mg/day fingolimod, 1.25mg/day fingolimod, or once-a-week intramuscular interferon-B1a for 12 months.3 The annualized relapse percentage was 0.16, 0.20, and 0.33, respectively.
Macular edema was a prominent adverse event reported in these and prior studies of fingolimod. Thirteen of 2,564 (0.5 percent) patients treated with fingolimod in these studies developed macular edema.1 In the TRANSFORMS study, four of the six patients found to have macular edema were taking a dosage of 1.25mg/d, andtwo were taking a dosage of 0.5mg/d. All seven patients in the FREEDOMS study found to have macular edema were taking a dose of 1.25mg/d. Macular edema was found to be dose dependent in the studies, with the frequency of FAME at the FDA-approved dosage of 0.5 mg/day, of only 0.2 percent.
The proposed mechanism behind FAME is loss of sphingosine 1-phosphate receptor signaling in endothelial cells, leading to downregulation of adhesion complexes, and enhanced vascular permeability.4 The increased vascular permeability causes a breakdown of the blood-retinal barrier and the result is macular edema.
Ophthalmic examinations
Per FDA recommendations, patients should undergo a baseline ophthalmic examination prior to initiation of fingolimod therapy. At the baseline examination, a complete ophthalmic examination, including dilated fundus examination, should be performed. An optical coherence tomography (OCT) would be beneficial to obtain at baseline but is not an absolute necessity. Amsler grid testing is an additional test that can be performed in office to screen for subtle macular changes. The eyecare practitioner should also discuss the signs and symptoms of macular edema with the patient. Further, patients should be warned to look for blurred vision, metamorphopsia, or a central shadowing of vision, consistent with the onset of macular edema. Additionally, home Amsler grid can be discussed and dispensed for patients to monitor symptoms at home.
Patients with a history of diabetes mellitus or uveitis are at a higher risk of FAME and should be informed of this at initiation of treatment.1 These pre-existing conditions, however, are not an absolute contraindication to treatment. Lastly, it should be advised against initiating fingolimod treatment within three months of intraocular surgery due to the increased risk of FAME during the post-operative period.
Repeat ophthalmic examination should be performed at three to four months, as most cases of FAME in the trials developed during this initial time period.2,3 On follow-up a dilated fundus examination should be performed and any patient with decreased vision, visual symptoms or changes on Amsler grid consistent with macular edema should have an OCT or fluorescein angiography for confirmation. OCT should reveal intraretinal cysts characteristic of cystoid macular edema, while angiography will demonstrate leaking within the macula. OCT or fluorescein can also be done at follow-up examination in the absence of symptoms or clinical findings on DFE at the clinicians discretion. Another follow-up exam is advisable at six months and annually thereafter. If patient have any changes in vision they should be advised to return to clinic sooner than their scheduled follow-up.
First step of FAME treatment
Discontinuation of fingolimod is the first step in the treatment of FAME. As with most cases of drug-induced macular edema, FAME usually resolves with cessation of the drug.2,3,5 In the TRANSFORMS and FREEDOMS studies, CME took up to six months to resolve after cessation of the drug. In a more recent study,6 FAME was managed successfully with newer generation topical non-steroidal anti-inflammatory drugs and topical steroids. In this study, macular edema resolved in two patients within four weeks with the use of topical non-steroidal anti-inflammatory drugs.7 In one case, macular edema resolved with topical non-steroidal anti-inflammatory drugs and a topical steroid even with the continued use of fingolimod. This is noteworthy for FAME patients who chose to continue with fingolimod therapy, whether due to minimal visual symptoms of FAME or good neurological control of MS that outweighs visual symptoms. Further clinical trials may shed light on the optimal management of FAME with and without cessation of fingolimod.
The 0.5 percent incidence of FAME in the TRANSFORMS and FREEDOMS trials may be under-representative because time-domain OCT was utilized in the aforementioned studies as opposed to the more sensitive spectral domain-OCT. Additionally it is expected that the number of cases of macular edema may increase as fingolimod use rises and long-term follow-up expands. Further investigation of FAME continues as part of an ongoing Phase 3 study (FREEDOMS II) that explores FAME with serial OCT scans in the first 300 participants.7
It is generally recommended that all patients on fingolimod be seen at baseline, and again at three and six months during the first year of treatment. Further, in any patient with macula edema, medications should be reviewed to see if the edema may be associated with this medication, and a referral placed back to the prescribing physician to see if discontinuation is advised. Although the incidence is somewhat rare, it is an important adverse reaction that both the clinician and patient should be aware.ODT
References
1. Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence detection and management. Neurology. 2012 Feb 28;78(9):672-80.
2. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010 Feb 4;362(5):402-15.
3. Kappos L, Radue EW, O’Connor P, et al. A placebo controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010 Feb 4;362(5):387-401.
4. Oo ML, Chang SH, Thangada S, et al. Engagement of SIP1-degrradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011 Jun;121(6):2290-300.
5. Turaka K, Bryan JS. Does fingolimod in multiple sclerosis patients cause macular edema? J Neurol. 2012 FEb;259(2):386-8.
6. Afshar AR, Fernandes JK, Patel RD, et at. Cystoid macular edema associated with fingolimod use for multiple sclerosis. JAMA Ophthalmol. 2013 Jan;131(1):103-7.
7. Hariprasad SM, Akudman L, Clever JA, et al. Treatment of cystoid macular edema with the new generation NSID nepafenac 0.1%. Clin Ophthalmol. 2009;3:147-54.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.