New cyclosporine therapy joins fight against dry eye disease
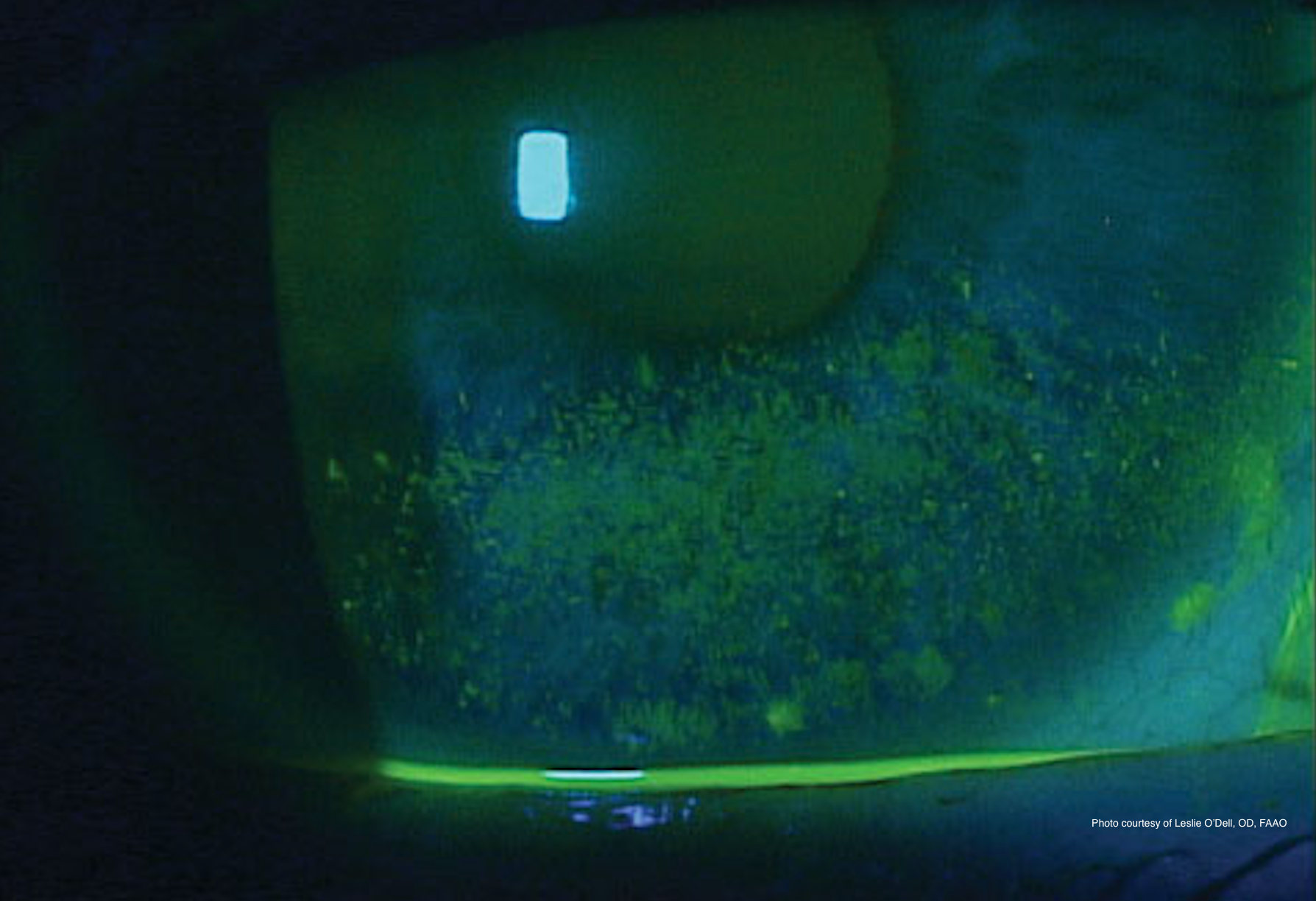
Dry eye disease has recently been redefined as a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms.1
Tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1
In an effort to curb these symptoms, new research on cyclosporine therapy shows promising results that may change the landscape of treatment for the better.
Cyclosporine
Cyclosporine is an immunomodulatory agent found in soil fungi (Beauveria nivea). Topical 0.05% cyclosporine (Restasis, Allergan) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of keratoconjunctivitis sicca-dry eyes-in April 2003.
Research shows its efficacy for both clinical signs of dry eye disease as well as symptoms. Cyclosporine has been shown to block T-cell proliferation, an inflammatory response contributing to dry eye disease.2
More recently, the introduction of Restasis Multidose has allowed for preservative-free dosing of cyclosporine 0.05% with the convenience of a bottle.
One of the most impressive findings has been cyclosporine’s ability to improve goblet cell density. Loss of goblet cells is known as an early cause of dry eye due to the increased levels of inflammatory cytokines as well as the reduction of mucin contributing to tear film instability and loss of homeostasis.3
Research by Stephen Pflugfelder, MD, showed that cyclosporine 0.05% increases goblet cell density and production of the immunoregulatory factor TGF- β2 in the bulbar conjunctiva in patients with dry eye.4
Generic
While generic cyclosporine 0.05% is looming on the horizon, it does not always mean a lower cost to the patient. For patients using Restasis in the single-use vials, there is a potential for the pharmacy to change the patient to generic without an OD’s request. However, the potency of generic medications is always a concern.
In response to the increasing cost of medications, compounded pharmacies and compounded medications are also on the rise. Imprimis Rx produces a compounded cyclosporine 0.05% for a fraction of the cost, despite the fact that many patients pay for compounded out of pocket.
Due to the change in formulation with a vehicle different than Refresh Endura (Allergan), patients often complain of instillation site irritation.
Cequa makes its debut
Sun Pharma has received approval for Cequa (cyclosporine ophthalmic solution) 0.09%, from the FDA. The drug is indicated to increase tear production in patients with keratoconjunctivitis sicca.
Cequa is the first and only approved cyclosporine product that incorporates a nanomicellar technology. This small molecule technology allows for better penetration of the active molecule into the ocular tissues.5
After 12 weeks of treatment in the Phase 3 confirmatory trial on Cequa,the product showed statistically significant improvement in the primary endpoint, Schirmer’s test (a measurement of tear production) (p<0.01). Improvements in secondary endpoints, like ocular staining assessments, were seen as early as one month after initiating treatment.6
Results
Cequa is dosed twice daily and will be available as a single-use vial. The most common adverse reaction following the use of cyclosporine ophthalmic solution 0.09% was instillation site pain (22 percent) and conjunctival hyperemia (6 percent). 6
Other adverse reactions reported in 1 to 5 percent of the patients were eye irritation, blepharitis, urinary tract infection, headache, and bronchitis. 6
Clinical studies showed a higher percentage of eyes treated with Cequa over vehicle to have Schirmer scores increase >10mm from baseline by Day 84.6
This was found in 16.8 percent of those treated with Cequa vs 8.6 percent using vehicle. Prior studies for the approval of Restasis showed an increase in Schirmer scores of 10mm in 15 percent of patients vs. 5 percent using vehicle at six months. 6
Wrapping up
In the end, it’s about finding the best treatment for the patient. With dry eye, it’s important to reach for a medication to address the inflammatory component of dry eye over simply recommending an artificial tear supplement.
As advancements in medications and formulations continue to grow, there will continue to be more options to improve patient care.
References:
1. Tear Film and Ocular Surface Society DEWS II - Definition and Classification. Available at: https://www.tfosdewsreport.org/report-definition_and_classification/48_36/en/. Accessed 10/12/18.
2. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Archives of ophthalmology. 2012;130(1):90-100.
3. Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr Eye Res. 2001;22:8-18.
4. Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta 2 production. Cornea 2008;27:64-9
5. Vaishya RD, Khurana V, Patel S, Mitra AK. Wiley interdisciplinary reviews: Nanomedicine and nanobiotechnology. Controlled ocular drug delivery with nanomicelles. 2014;6(5):422-437. doi:10.1002/wnan.1272.
6. Cequa [package insert]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210913s000lbl.pdf. Accessed 10/19/18.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.