Recall of brimonidine tartrate ophthalmic solution voluntarily issued by Apotex Corp.
This recall is being initiated out of an abundance of caution due to cracks that have developed in some of the units caps of brimonidine tartrate ophthalmic solution bottles

With guidance of the US FDA, Apotex Corp. has initiated a voluntary recall at the consumer level for 6 lots of brimonidine tartrate ophthalmic solution, 0.15%, distributed nationwide in the United States between April 5, 2022 to February 22, 2023.1 Brimonidine tartrate ophthalmic solution is an alpha-adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
This recall has been initiated out of an abundance of caution due to cracks that have developed in some of the units caps of brimonidine tartrate ophthalmic solution bottles, which could impact sterility of the solution.
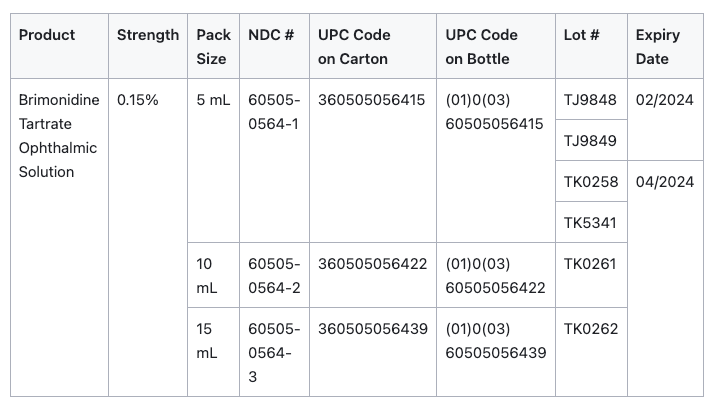
The six (6) lots of Brimonidine Tartrate Ophthalmic Solution, 0.15% can be identified by NDC numbers stated on the carton and label of the product. The lot number and expiry date are located on the top flap of the carton and to the left side of the product description on the bottle label beside the barcode. These lots were distributed nationwide in the USA between April 05, 2022 to February 22, 2023.
Chart courtesy of Drugs.com.1
All impacted direct accounts (wholesalers, distributors, warehousing chains, mail order pharmacies, and long-term care pharmacies) are being notified by Apotex Corp. via email and mail, and the return of all recalled product is being arranged. Patients who have received the identified lots or have questions regarding this recall should contact their pharmacy, as well as immediately contact their health care provider for medical advice and return the identified lots to Inmar Rx Solutions by calling 1-855-275-1273 (9:00am – 5:00pm, EST Monday thru Friday).
Consumers with questions regarding this recall can contact Apotex Corp. by phone at 1-800-706-5575 (8:30am – 5:00pm, EST Monday thru Friday) or email address UScustomerservice@Apotex.com. Consumers should contact their physician or health care provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
References
1. FDA alert: Apotex Corp.. issues voluntary nationwide recall of brimonidine tartrate ophthalmic solution, 0.15% due to cracks that have developed in some of the units caps of the bottles. Drugs.com. http://bit.ly/41Qk0JG.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.