Retinal vein occlusion care center enters new era
Positive results from recent studies evaluating intravitreal steroids and anti-vascular endothelial growth factor agents for the treatment of retinal vein occlusions have resulted in FDA approvals for two products and may be expected to shape a new standard of care for these vision-threatening disorders.

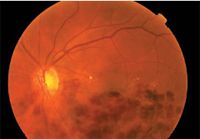
"Now, the outcomes from large-scale prospective studies proving the success of intravitreal corticosteroids and ranibizumab (Lucentis, Genentech) have the potential to revolutionize treatment for RVOs," she added.
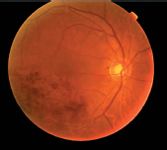
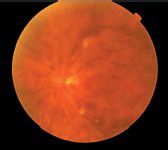
Novel intravitreal corticosteroid
The primary efficacy endpoint in this study was time to achieve a ≥15 letter increase in best corrected visual acuity (BCVA), and the two dexamethasone groups demonstrated statistically significant superiority compared with controls for both patients with BRVO and CRVO.
"In this study, a statistically significant difference favoring dexamethasone in the proportion of patients reaching this endpoint was present between days 30 and 90, and the rate peaked about day 60 at 29%. These data suggest perhaps greater benefit could be achieved with earlier retreatment," Dr. Lim said.
Shorter durations of macular edema were associated with greater improvements in visual acuity in the dexamethasone treatment groups. The average reduction in central retinal thickness was significantly greater in both dexamethasone treatment groups than with sham at day 90, but this effect did not persist to day 180.
The intravitreal dexamethasone implant was associated with IOP elevations, but the increases peaked at day 60 and the mean IOP was not significantly different from the sham group at day 180. The corticosteroid was also associated with cataract formation, but the overall incidence was not statistically different between the dexamethasone versus sham treated groups.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.