SECO 2023: Pipeline update from Tarsus
Tarsus has received a PDUFA date for their TP-03 drug, which they are hoping to bring to market later this year.
Aziz Mottiwala, Chief Commercial Officer for Tarsus Pharmaceuticals, shares a pipeline update while catching up with Optometry Times® during the 100th anniversary SECO meeting held this year in Atlanta, Georgia.
Editor's note: This transcript has been edited for clarity.
Mottiwala:
Hi, I'm Aziz Mottiwala, the Chief Commercial Officer at Tarsus. And we're delighted to be here at SECO meeting, being able to provide some great updates to the eye care community about our work in Demodex blepharitis, and potentially bringing TP-03 to the market later this year.
We filed the drug last year with the FDA and we now have a PDUFA date of August 25, 2023. Meaning if all goes well, we could potentially have a product in the hands of clinicians later this year.
And it's important because we're going to be able to help those doctors serve up to 25 million patients in the US—that's almost half the patients that are coming into an eye care clinic today.
These are patients that have been diagnosed with blepharitis that don't have a good solution, patients that may have dry eye that have been misdiagnosed or have concomitant disease, and patients that are coming in for cataract surgery or can't stand their contact lenses.
We've gotten a lot of great feedback from the eye care community, so we're doing all the things we need to do to prepare for that launch. We've really focused on three things.
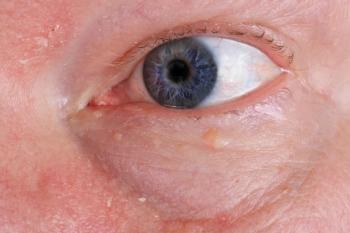
One is building a world class team, and it's a team that brings the best of eye care expertise and product launching category creation experience to the table. And that team is working on educating the market. We recently launched the "Look at the Lids" campaign, which encourages every doctor to start an eye exam by looking at the lids, taking just a few seconds to look for collorettes—that telltale sign of Demodex blepharitis to help identify these patients so that when a drugs available, they can treat them.
We've also launched an MSL team, a team that is comprised of all optometrists, it's something very proud of, of having some of the brightest ODs in the marketplace, on our team helping to educate their colleagues about the disease.
Beyond that, we're working with payers to ensure that when the product is available, that it's affordable and accessible.
And lastly, we continue to get great feedback and engagement from the eye care committee. The feedback, advice, and insights we get are really helping shape our strategy.
And we look forward to continuing to provide updates to the eye care community and getting this drug into patients' hands later this year. Thank you.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.