A stepwise approach to diagnosing MGD
Focusing on dry eye management is a great practice builder, but is not without challenges. These challenges lie in making the proper diagnosis, implementing new technology, properly training staff, developing an effective treatment plan and the time it takes to properly educate patients.

Focusing on dry eye management is a great practice builder, but is not without challenges. These challenges lie in making the proper diagnosis, implementing new technology, properly training staff, developing an effective treatment plan and the time it takes to properly educate patients.
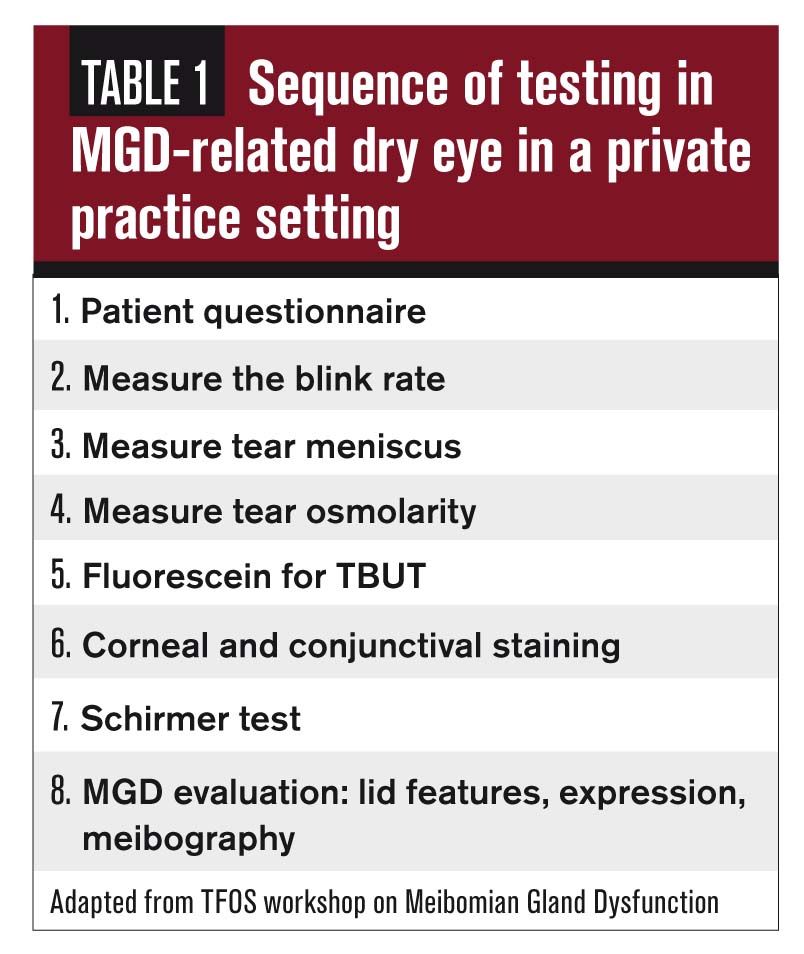
When facing any challenge, establishing and following a set protocol helps. The Tear Film and Ocular Surface Society (TFOS) Workshop on meibomian gland dysfunction (MGD) helped to organize the evaluation of a patient when MGD is suspected, listing the appropriate tests needed for proper diagnosis.1 (Table 1)
Let’s go through each one and create a step-by-step approach to a vast and complicated disease, MGD. For all testing, be sure to develop a standard of care that is universal for every patient encounter to track improvement during subsequent exams.
Related: Using warm compresses to treat MGD
Understanding the definition of MGD is the foundation. Through the work of
TFOS, MGD was formally defined as a “chronic, diffuse abnormality of the meibomian gland, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.”1
Step 1: History
Questionnaires can expedite your history intake. These can be validated surveys, such as the Ocular Surface Disease Index (OSDI) and Standard Patient Evaluation of Eye Dryness (SPEED) questionnaires, as well as customized questionnaires. Here are a few important questions to ask for all patients-symptomatic or not.
• How do your eyes feel in the morning when you are waking? For some patients, morning complaints clue us into other potential problems with demodex blepharitis, nocturnal lagophthalmos, and recurrent corneal erosion high on the list of differentials.
• How long can you read or use a computer before your vision blurs or you become aware of your eyes?
More from Dr. O'Dell: Using the SPEED questionnaire to identify dry eye
Because a lot of patients coming in for an exam are already using an over-the-counter drop or have tried one, also ask if the drop she is currently using provides some relief to her symptoms.
For a contact lens patient, the questions can seem endless:
• What brand of lens is he wearing?
• What’s the replacement schedule for the lenses?
• How compliant is he to replacement and cleaning?
• What does he use for disinfecting his lenses?
• How often does he sleep in lenses?
• Is he aware of the lens during the day?
• Is his vision stable even with blinking?
Related: Why the tear film matters
When reviewing medications and other systemic conditions that contribute to MGD, ask if the patient is also experiencing dry mouth symptoms. For many dry eye sufferers, taking the time to talk about their symptoms is like opening Pandora’s box. Once you have a chance to take back over control of the conversation, you can start your examination.
Next: Take a step back
Step 2: Take a step back
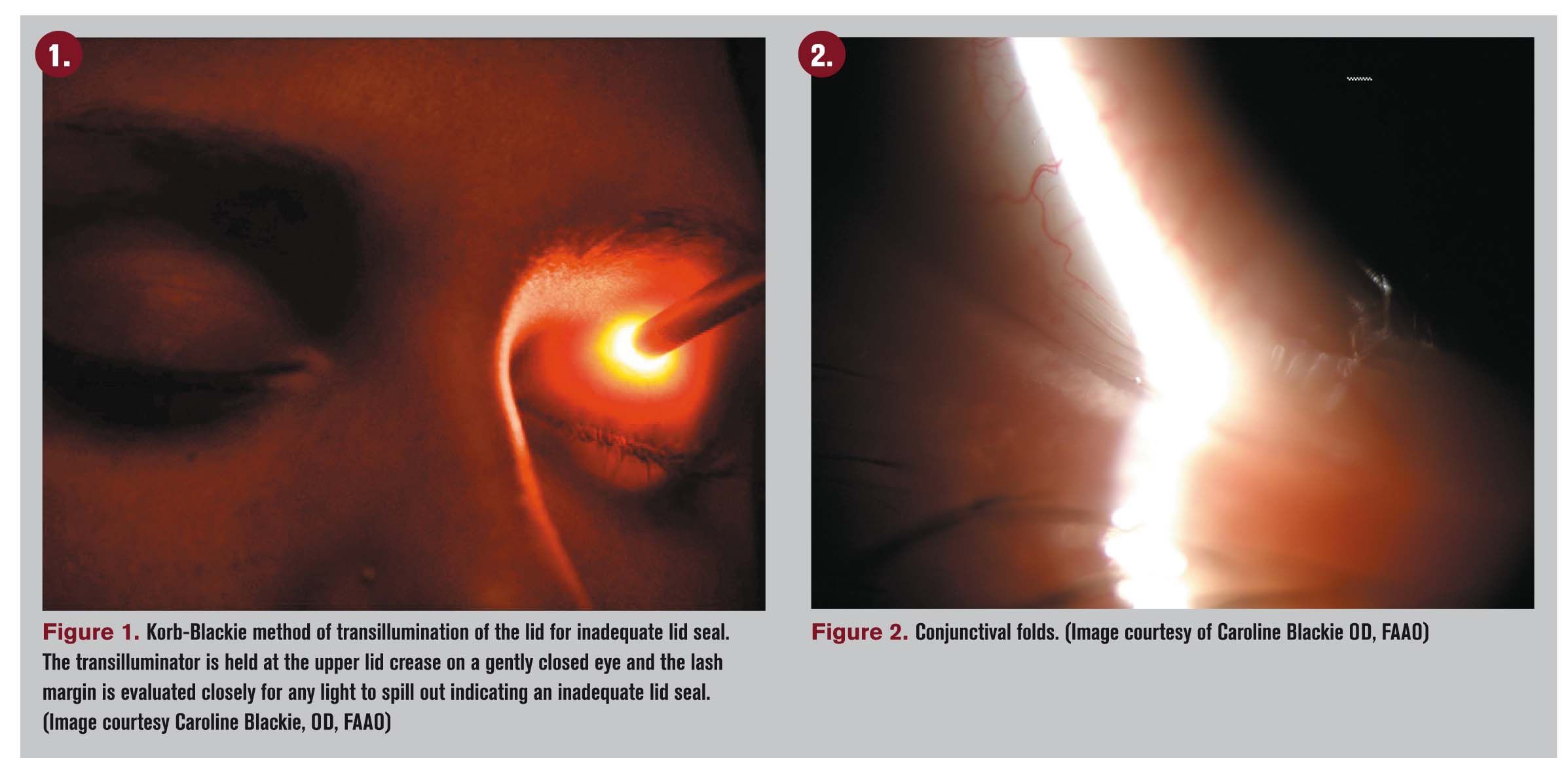
Before a slit lamp evaluation, take a step back from the patient to evaluate her external appearance. Look for signs of rosacea, abnormalities to the lid-globe congruity, blink rate, and overall appearance of the eye.
Next, pick up your transilluminator and evaluate the patient for inadequate lid seal. Ask the patient to close her eyes as if she were resting-no forced closure-and rest the transilluminator on the top eyelid (Figure 1). Evaluate the lid for inadequate lid seal centrally, nasally, and temporally. Then start your slit lamp evaluation.
Related: Diagnosing and treating dry eye with technology
Step 3: The bread and butter of MGD: slit lamp examination
Through research studies, I have developed superior techniques for using vital dyes to best determine corneal and conjunctival staining as well as lid wiper epitheliopathy.
A formal dry eye strip can be used to measure tear break-up time (TBUT) and is applied to the superior conjunctiva with the patient looking down. This applies a very thin amount of fluorescein. Average TBUT for three blink cycles per eye and record.
If you don’t have time for this or access to dry eye strips, use a fluorescein strip applied superiorly and record it in a way that is the same for every patient encounter; simply stating “decreased TBUT” or “instant TBUT.”
The next step is to instill fluorescein dye using a strip. This is best applied inferiorally and temporally while the patient is looking up. It’s important to wait 60 to 90 seconds after instillation before evaluating the cornea for staining. During that time, bring the patient to the slit lamp and start evaluating his lid and lash margin for blepharitis or demodex.
Instruct the patient to open his eyes and observe the bottom lid margin looking for obvious changes to the meibomian glands. You are looking for cicatricial changes of the glands, telangetatic vessels, meibomian gland cyst and/or a frothy tear film.
Next, observe the conjunctival tissue adjacent to the cornea both nasally and temporally looking for conjunctivochalsis, a bagginess to the episclera that can interfere with tear distribution as well as a continuous feeling of “my eyes are tearing” (Figure 2). Also, take this time to evaluate the tear meniscus height when enhanced with fluorescein dye. Once the wait time is up, the cornea can be evaluated for keratitis.
More on dry eye: 5 things you don't know about punctal plugs

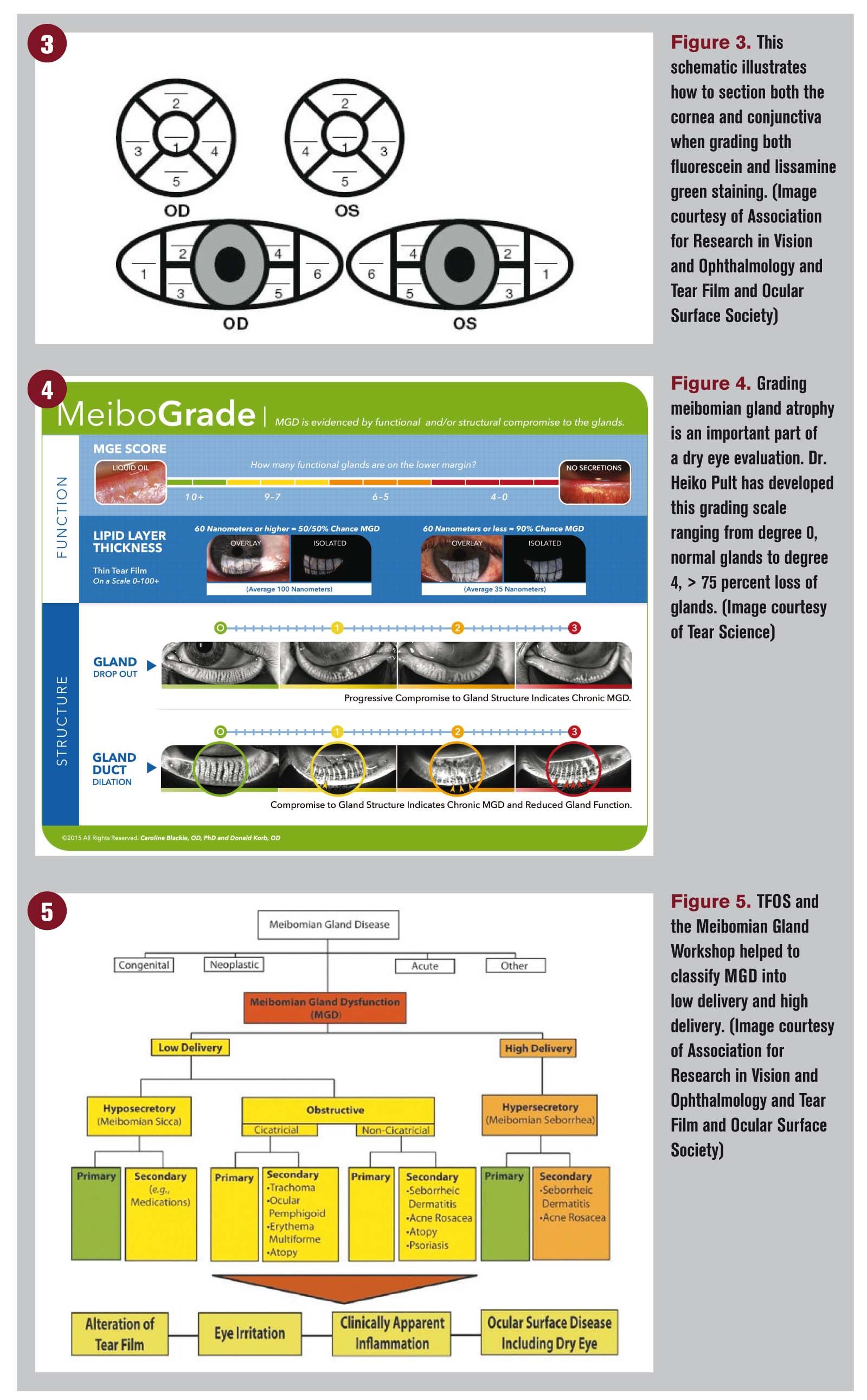
Using a standardized grading system will help when you follow the patient. I recommend looking at the cornea broken into five segments: central, superior, temporal, inferior, and nasal. Then grade superficial punctate keratitis (SPK) in each segment using a grading scale of Grade 0 to 3 where zero is no staining.2 (Figure 3).
The location of keratitis helps to guide the diagnosis as well as best treatment plan.
Now add lissamine green stain using the same method as fluorescein, adding dye inferiorly and waiting 60 to 90 seconds before evaluating the conjunctiva for staining. During this time, start to evaluate meibomian gland secretions.
This is best done using the Korb Meibomian Gland Evaluator developed by Dr. Donald Korb (TearScience). This gland evaluator provides a standardized pressure comparable to the pressure exerted with blinking to determine how many glands are secreting.3
With the patient looking upward, gently push on the bottom lid and section the glands in groups of five, grading the expressibility of the glands first on a scale of 0 for no secretion to 3 for easy secretion and thin healthy oil.
When the time has lapsed for the lissamine dye, evaluate the conjunctival tissue looking for staining again on a scale of Grade 0-3 in the nasal and temporally quadrants. Again, using a standardized scale allows for easy documentation as well as uniformity in your charts to know when patients are getting better from your treatment or worse.
Next: MGD must-haves
Step 4: MGD must-haves
There are a few essentials to an MGD evaluation that if you aren’t doing presently, you should add to your exam today. All are easy and don’t cost you a dime to add.
First, transillumination of the MG using a penlight at the slit lamp. This is a quick and easy method to evaluate for MG atrophy.
Again, a grading scale should be used; a preferred grading scale was developed by Dr. Heiko Pult and is a great education tool for the patient as well4 (Figure 4).
A tool to express the glands in another must-have in a MGD evaluation. Being able to express the glands allows for grading of inspissation and obstruction. There are a few commercially available, some with flat metal plates, some with barrels that roll over the glands to aid in expression.
The evaluation of a dry eye patient now has diagnostic testing available to enhance our clinical skills and improve our ability to diagnose a patient. These include tear osmolarity (TearLab), InflammaDry (RPS), meibography (Oculus), interferometry (TearScience) and external photography and videos.
These tests enhance patient education-seeing is believing for our patients.
Think of our glaucoma patients. If we practiced in the days prior to optic nerve analysis, we could still diagnose a patient with glaucoma based on observations to their nerve fiber layer.
The new diagnostics available with OCT and HRT improve our ability for early detection, but an optic nerve evaluation is still needed to correlate all the tests. The same is true with advancement in diagnostics for dry eye. A strong dry eye clinician can use these data points to develop a patient-centered treatment plan.
Related: New technology could replace eye drops

Step 5: Diagnosis
The next challenge is changing the way we document this disease. Consistent nomenclature is imperative, and we as a profession need to use the recommendations laid out by the MGD workshop.5
Meibomian gland dysfunction can be classified as either low delivery (hyopsecretory/obstructive) or high delivery (hypersecretory) (Figure 5).
Making the right first diagnosis based on a complete history and examination with the proper testing using technology available within your practice sets the foundation for treatment.
References:
1. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1922-9.
2. Barr JT, Schechtman KB, Fink BA, et al. Corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: baseline prevalence and repeatability of detection. Cornea. 1999 Jan;18(1): 34-46.
3. Korb DR, Blackie CA. Cornea. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008 Dec; 27(10): 1142-7.
4. Pult H, Riede-Pult BH, Comparison of subjective grading and objective assessment in meibography. ̢۬Cont Lens Anterior Eye. 2013 Feb;36(1):22-7.
5. Nelson JD, Shimazaki J Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1930-7.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.





.png&w=3840&q=75)











