Visus Therapeutics launches phase 3 trials for presbyopia eye drop
The latest launches of BRIO-I and BRIO-II follow promising topline data from the phase 2 VIVD clinical study evaluating Brimochol PF.
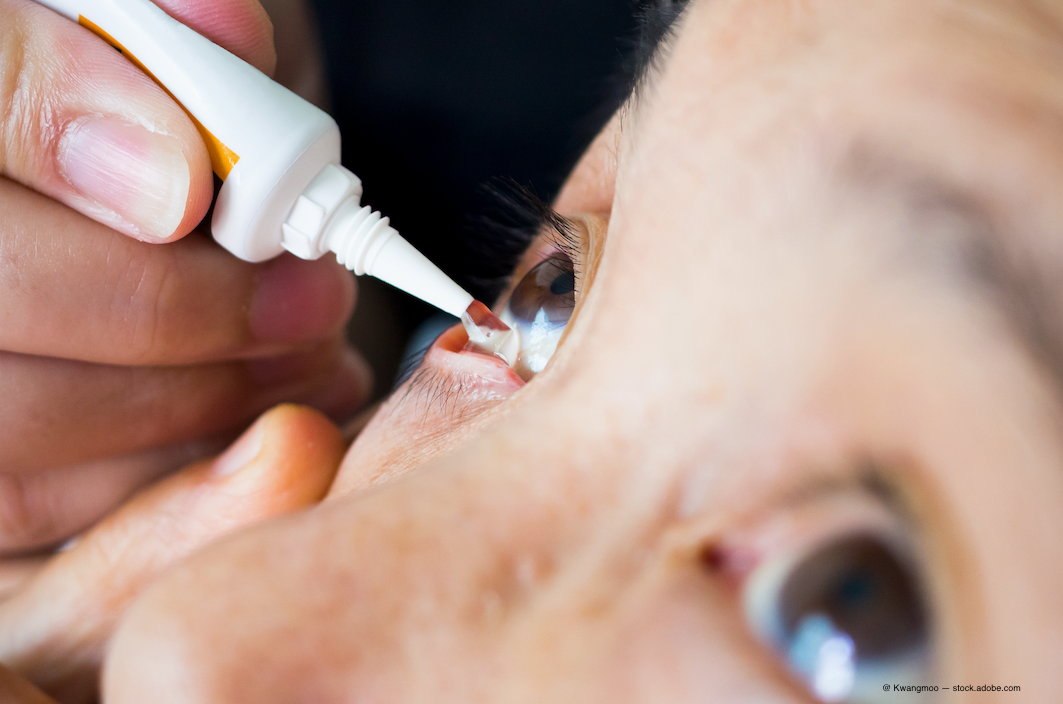
Visus Therapeutics has begun the first of two pivotal phase 3 trials for its lead product, Brimochol PF, a preservative-free topical ophthalmic solution for the treatment of presbyopia, the company announced Tuesday.
The trials, BRIO-I and BRIO-II, are double-masked, randomized, multi-center, safety, and efficacy studies are expected to enroll an estimated 170 to 500 patients who are emmetropic phakic and pseudophakic presbyopic, according to a news release.
Both formulations create a pinhole effect in the eye that reduces the pupil and allows light rays focused on the retina to enter the eye, resulting in sharpened vision and mitigated side effects.
The latest launches follow promising topline data from the phase 2 VIVD clinical study.
Results demonstrated that Brimochol PF and Carbachol PF, two preservative-free formulations, were well tolerated and met clinical outcomes to continue advancements into pivotal trials, the company stated.
Visus Therapeutics intends to present Brimochol PF, which is a fixed-dose combination of carbachol and brimonidine tartrate, as a safe and effective once-daily eye drop for the treatment of presbyopia-related vision loss.
Rhett Schimman, MD, MS, MHSA, co-founder, chief medical officer and head of research and development at Visus Therapeutics, said in a statement that the response rates of the formulations appear to be much higher over an extended period of time than the commercially available therapy as well as the therapies in development, according to clinical studies.
“We anticipate the Phase 3 trial will generate the pivotal data required to support our New Drug Application, bringing us one step closer to offering a novel, preservative-free therapy for patients,” Schimman said, in a statement.
The primary efficacy endpoint is the percentage of patients who gain three lines of improvement in binocular near visual acuity without losing one line of distance vision, according to the release.
A first read-out of the study is expected by the fourth quarter of 2022.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.