More than a yellow lesion?

A 64-year-old Caucasian male presented for a comprehensive eye exam with chief complaint of mild blur at distance and near with his progressive addition lenses as well as a peeling anti-reflective coating.
Seems innocent, right? Let’s take a deeper look as the case unfolds.
Case history
The patient’s medical history was significant for hypertension, high cholesterol, and generalized anxiety for which he was taking amlodipine and simvastatin (Zocor, Merck), while alprazolam (Xanax, Pfizer) was administered as needed.
Remarkable surgical history was pertinent for a hernia repair. He reported an allergy to aspirin, resulting in cramps.
Related:
Clinical findings and management
His blood pressure was measured as 102/70 mm Hg at 2:31 p.m. with a height of 5’10” and weight of 165 pounds, resulting in a normal BMI of 23.7. He reported regular exercise and did not smoke.
Color vision was normal in each eye as well as stereopsis along with all other entrance testing. Of note, there was a small hyperopic and astigmatic shift in each eye, resulting in best corrected vision of 20/20.
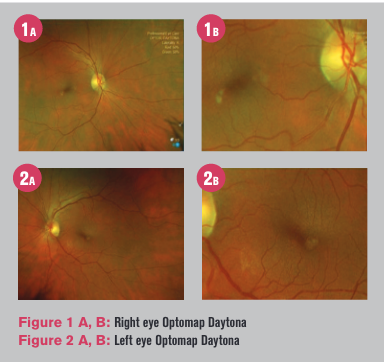
Intraocular pressure and anterior segment were unremarkable other than mild cortical cataracts. The posterior segment was also unremarkable, except for a 0.25 disc diameter-sized yellow lesion that happened to be inferotemporal parafoveal in each eye (Figures 1 and 2).
Noting this finding and what appeared to be soft confluent drusen and pigment mottling OD, the patient was advised to return for dark adaptation and ocular coherence tomography (OCT) OU.
Related: How inflammation may play a role in retinal disease
Dark adaptation (AdaptDx MacuLogix) testing was reliable (<30 percent fixation errors) but delayed in the right eye with a rod intercept of 11.41 minutes and normal in the left eye at 3.46 minutes.
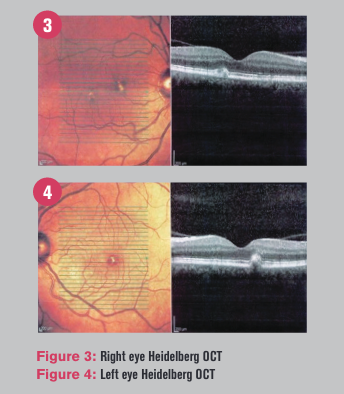
Macular OCT revealed a sub-retinal pigment epithelium (RPE) elevation in each eye as well as classic subtle deflections evident of drusen and vitreomacular adhesions (Figures 3 and 4).
The patient then underwent Macula Risk (ArcticDx) genetic testing via cheek swab.
Results noted nine out of 15 genetic markers as high risk, classifying the patient as MR4 with a 6 percent, 16 percent, and 32 percent likelihood of developing advanced age-related macular degeneration (AMD) in two, five, and 10 years, respectively (Figure 6).
The patient was asked to return to discuss the results, which also specified which supplement he should be taking to reduce his risk of progressing to advanced AMD. There was no family history of AMD, which can increase the odds of a patient having AMD by 27.8 times with an affected parent or 12 times with an affected sibling.1Related: Why doctors are rethinking AMD standards
Discussion
Differential diagnoses of yellow lesions in the macular area include large drusen, epiretinal membrane, pseudohole, lamellar macular hole, Stargardt disease, chronic central serous chorioretinopathy, and Best’s vitelliform macular dystrophy. In this case, focal presentation and clinical signs via OCT suggest drusenoid pigment epithelial detachment (PED) or adult-onset vitelliform macular dystrophy (AVMD).
AVMD is an early-onset autosomal dominant disorder in which accumulation of lipofuscin-like material within and beneath the RPE is associated with progressive loss of central vision.2
The timing of AVMD usually begins after age 40, leading me to believe this patient’s presentation represents an early-stage lesion.
The findings are highly autofluorescent and will become larger in later stages, with central clearing of the yellowish substance along with deposition of autofluorescent subretinal material at the outer borders of the lesion.3Related: Vision loss presents potential complications
As review, Macula Risk PGx tests 15 genetic markers across 12 AMD associated genes. It has shown a predictive accuracy of 89.5 percent with sensitivity and specificity >80 percent.4
Taking into account that this patient has a high likelihood of converting to advanced AMD, clinical monitoring every six months and home monitoring with Amsler grid is prudent. Other home monitoring technologies include ForeseeHome (Notal Vision).
Additionally, a baseline threshold central 10-2 visual field to establish the sensitivity of the AVMD lesions and the mean deviation would be valuable to perform. Microperimetry may also be relevant in the future, but it is not medically necessary at this stage.
Conclusion
While this patient had AVMD and early-stage dry AMD, it cannot be understated that the loss of photoreceptor function secondary to AMD can be evaluated by dark adaptation and may precede obvious signs of drusen by up to three years.
Read more retina content here
References:
1. Shahid H, Khan JC, Cipriani V, et al. Age-related macular degeneration: the importance of family history as a risk factor. Br J Ophthalmol 2012;96(3):427-31.
2. Sun H, Tsunenari T, Yau KW, et al. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A 2002;99(6):4008-13.
3. Spaide RF, Noble K, Morgan A, et al. Vitelliform macular dystrophy. Ophthalmology 2006;113(8):1392-400.
4. Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53(3):1548–1556.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.