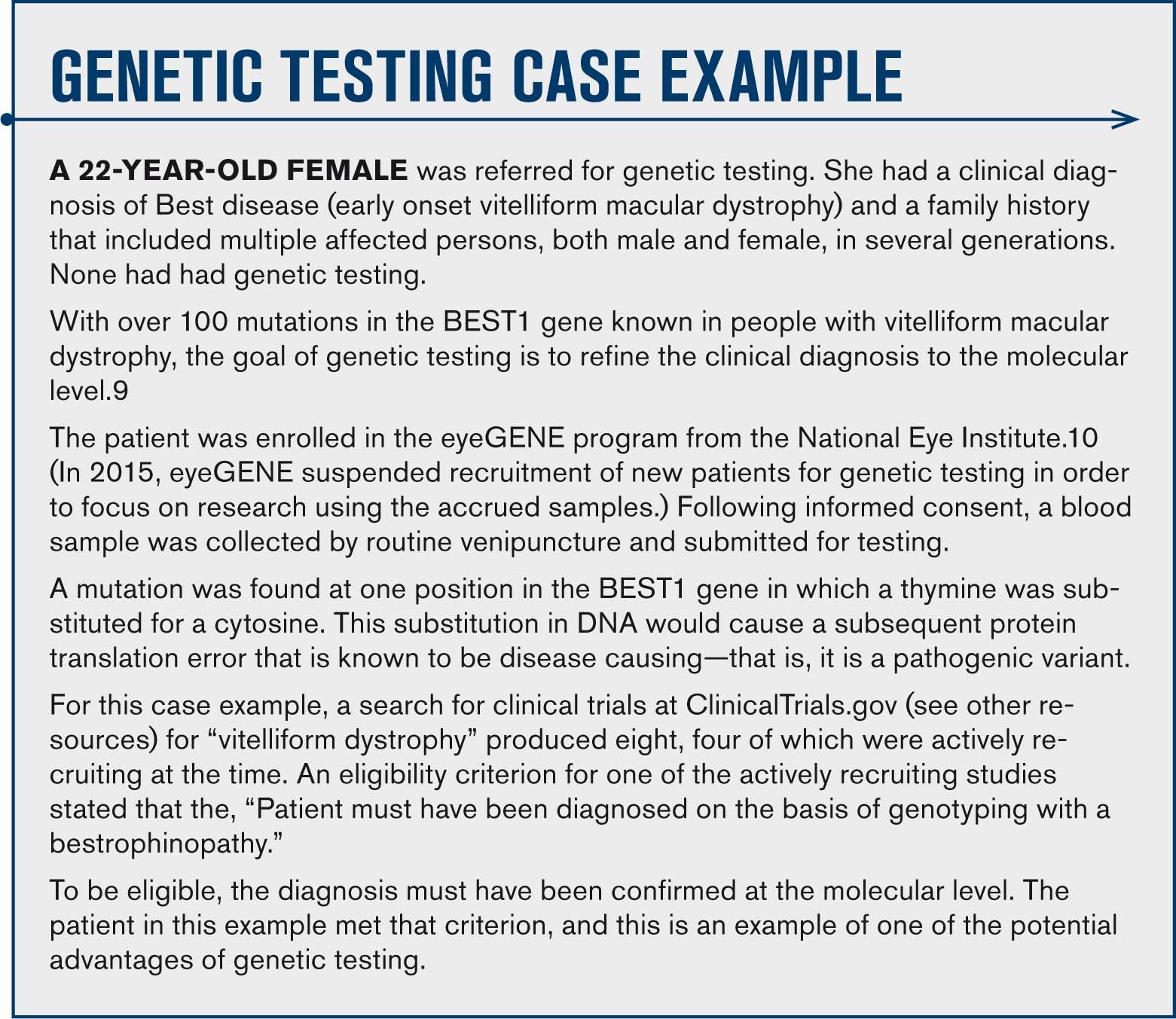
Understanding genetic testing for hereditary disease
Genetic testing is becoming more successful and is expanding in scope. Understand how the process works and how to guide your patients through it. Find out what is involved and how to manage genetic testing…plus access a list of resources for you and your patient.


Diagnosis at the molecular level is quickly becoming standard of care. The national initiative in precision medicine will rely heavily on genetic diagnoses by taking into account “individual differences in people’s genes.”1
Gene therapy and genetic-based trials are increasing rapidly in success and number and are expanding in scope and concept. Gene therapy with Luxturna (Spark Therapeutics) for Leber congenital amaurosis received FDA approval December 19, 2017,2 and the first patient was treated on March 20, 2018.3 Eligibility for treatment includes biallelic mutations in the RPE65 gene confirmed by genetic testing.
It is incumbent on the practitioner to recognize potentially hereditary eye disease and discuss referral for genetic testing. Realize that not every patient will want genetic testing.
Two concerns with genetic testing are protecting privacy and-related to that-avoiding possible discrimination by employers. Title II of the Genetic Information Nondiscrimination Act of 2008 (GINA) became effective in 2009 and prohibits employment discrimination based on genetic information.4 This fact might reassure some patients, but others will have different concerns; they should know that genetic testing can be performed later if they change their minds. Consultation with a genetic counselor or medical or clinical geneticist might help them with this decision without committing them to testing.

Molecular level diagnosis
Retinitis pigmentosa serves as a good example of why diagnosis at the molecular level is important. In 2013, Daiger et al reported that over 3,100 known mutations, within about 50 genes, cause what we call retinitis pigmentosa clinically.5 That means over 3,100 different diagnoses could be made, and it is no longer sufficient to diagnose “retinitis pigmentosa” without offering genetic testing that could refine the diagnosis to the molecular level.
“Could” is the operative word because we may test and find nothing. If there are 3,100 known mutations, maybe there are actually 6,100 but we don’t know the other 3,000 so those may not show up with current test strategies. Or, if there are 50 known genes, maybe there are another 50 we don’t know about yet and hence may not get tested. These are overly simplistic examples, but it adequately conveys the concern that we may test someone with clinical retinitis pigmentosa but not find a causative gene mutation.
Related: Current views on genetic testing for AMD
Gene or mutation-based therapies are being investigated and are having some success. Eligibility criteria for various clinical trials often states that candidates must have a confirmed diagnosis through genetic testing-another good reason for testing. Because results from genetic testing can take two to three months, it seems reasonable for a patient to get testing done so he has that information when or if a clinical trial comes along that interests him. The best resource for discovering what types of clinical trials are taking place is ClinicalTrials.gov (see “Genetic testing resources for ODs and patients” box).
Some, including third-party payers, may argue that genetic testing has little applicable value unless there is a direct connection to treatment; however, that is a very myopic view of what constitutes “treatment.”

The American College of Medical Genetics and Genomics (ACMG) points this out in a policy statement on the clinical utility of genetic and genomic services stating that: “…etiologic diagnosis prevents additional unnecessary testing, provides the opportunity for anticipatory guidance, and provides better information regarding recurrence risks for the family and the affected individual. Further, as increased numbers of individuals are diagnosed with specific genetic disorders, information will be obtained that will help predict future complications and risks, tailor medical interventions, and lead to the development of new specific therapies and management strategies.”6
Patient perceptions of the personal value of genetic testing that provided meaningful results from one study included four qualities:7

• Achieving a sense of empowerment over their own health
• Achieving a sense of legitimization of their suffering by no longer being a mystery or curiosity
• Achieving a sense of doing all that you can for your child, yourself, or family member(s)
• Achieving a sense of altruism by contributing to society to help others
Other patients have expressed relief at ending a “diagnostic odyssey” of multiple doctors’ visits and possibly unnecessary diagnostic testing, all with no answers and achieving hope where before there was none when a cause is found
What is involved
A patient interested in genetic testing will be referred to a medical or clinical geneticist experienced in eye disease. The evaluation will include a detailed history and construction of a family pedigree as well as a pathology-focused eye exam and additional testing to help determine what genetic test will be ordered provided that the patient wants to pursue it and gives informed consent.
Informed consent
Informed consent is an important first step. The patient needs to understand why testing is recommended and the potential outcomes-both pros and cons.
Related: How a newly-discovered gene affects myopia
The test order form will have one or more areas for the patient to give consent for testing, stating that she understands the reason for the test and possibly for allowing her anonymized DNA to remain in a repository to be used for research.
Testing is not necessarily benign, and there can be adverse effects. For example, it may confirm a diagnosis with a poor prognosis when the patient was hoping it would do the opposite. It could reveal non-paternity or an additional genetic variant that was not suspected. The patient should understand this before agreeing to testing.
Possible test outcomes
Any sequence alteration in DNA is usually reported as a “variant,” not as a “mutation.” This avoids the common misperception that a mutation is always disease-causing when in fact it might be benign.
It has been recommended that variants be reported with the following modifiers: pathogenic, likely pathogenic, uncertain significance, likely benign, or benign.8 These modifiers convey the important message that test results may carry a certain degree of uncertainty or even that nothing may be found. Again, it is important that patients understand these possibilities as part of the informed consent process prior to testing.

Sample collection
The sample collected is about 3-10 mL of blood from routine venipuncture of the arm. DNA is isolated from the white blood cells, and the DNA is tested. DNA can also be isolated from cells collected by a buccal smear or from cells collected from a special mouth wash. For infants, blood can be collected onto an absorbent paper following a heel stick with a sterile lancet.
Ordering the test
The desired test is ordered from a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory-and this is where it gets complicated. Testing requires a knowledgeable practitioner, typically a medical or clinical geneticist, because there are many different tests and the outcomes can be quite complex.
Related: Using OCTA in optometric practice
For example, one cannot order a “genetic test for retinitis pigmentosa.” Order forms are often lengthy, and laboratories may request a medical summary, family pedigree, and specific eye findings that will be reviewed to be sure the requested test is appropriate.

Genetic testing often takes 8 to 12 weeks but varies depending on what test is ordered. Insurance, with a few exceptions, typically does not cover genetic testing for hereditary eye disease.
Costs may vary from a few hundred dollars to a few thousand dollars. Cost also varies among laboratories, and some labs offer payment plans or fee adjustments based on financial need.
An experienced medical geneticist or certified and experienced genetic counselor can be helpful in sorting out the options.
Management
Genetic test results can be complex and difficult to interpret. The best practice is to review results face-to-face with the patient even if nothing was found.
My preferred procedure is to prepare a folder of information that the patient can take home. It contains a copy of the lab report, a summary of affected gene(s), and a summary of the disorder. Both summaries come from Genetics Home Reference (see other resources), which is an excellent source of information from the National Institutes of Health about all aspects of medical genetics.
The folder also includes a copy of the pedigree as determined at the initial clinic visit and a list of resources for further information. Each of these is reviewed in detail. The pedigree is reviewed to determine which, if any, family members might be at-risk to be a carrier or be affected but not yet diagnosed.
“Targeted” genetic testing can be offered to these individuals for the specific variant found in the initial patient. Targeted testing is usually less expensive because the lab already knows what to look for. This leads to a discussion of recurrence risk for the patient’s offspring, and it might be necessary to also test the patient’s parents before that can be assessed. Two other topics to address are prognosis and current or possibly future treatments, including gene therapy. All of this takes time and may require another visit.
The prospects of gene or mutation-based treatments including gene therapy are exciting, but meanwhile it is important to not overlook traditional aspects of management including maximizing vision with glasses or contact lenses, referral for low vision rehabilitation, referral for vocational rehabilitation services, as well as periodic eye exams to monitor progression and to not miss other ocular disorders.

Where we are headed
Diagnosis at the molecular level will likely become standard of care, and treatment with gene therapy may eventually have the potential to cure or restore substantial sight to those with many types of hereditary eye disease. Driving forces are the emphasis in precision medicine, advances in gene editing techniques, and the evolving success of gene or gene-based therapy
It seems likely that FDA-approved gene therapy for additional hereditary ophthalmic disorders will occur within the next several years. For many, eligibility for clinical trials and eventual treatments will begin with genetic testing.
Related: 6 things you need to know: Genetic markers for glaucoma identified
References
1. WhiteHouse.gov. The Precision Medicine Initiative. Available at: https://obamawhitehouse.archives.gov/precision-medicine. Accessed 3/27/18.
2. U.S. Food & Drug Administration. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm. Accessed 3/29/18.
3. Massachusetts Eye and Ear. Mass. Eye and Ear performs first FDA-approved gene therapy procedure for inherited disease. Available at: https://www.masseyeandear.org/news/press-releases/2018/03/mass-eye-and-ear-performs-first-fda-approved-gene-therapy-procedure. Accessed 3/29/18.
4. U.S. Equal Opportunity Employment Opportunity Commission. Genetic Information Discrimination. Available at: https://www.eeoc.gov/laws/types/genetic.cfm. Accessed 3/27/18.
5. Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013 Aug;84(2):132-41.
6. ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2015 Jun;17(6):505-7.
7. McCormick J, Halverson C, Clift K. Was it worth it? Patients’ views on the value of clinical genomic sequencing. (Platform Presentation in Whole Exome Sequencing), American College of Medical Genetics Annual Meeting, Tampa, FL, March 10, 2016.
8. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015 May;17(5):405-24.
9. Health conditions related to genetic changes. Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/gene/BEST1#conditions. Accessed 3/27/18.
10. National Ophthalmic Disease Genotyping and Phenotyping Network. About eyeGENE. Available at: https://eyegene.nih.gov/about. Accessed 3/27/18.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.
