Case report: the “other” AMD
Diagnosis highlights overlooked treatments for this disease
Figure 1. Baseline color fundus photographs of the right (L) and left (R) eyes. Note that there is a small area of healthy-looking macula in the right eye accounting for the visual performance. Figure 2. Baseline plus 2 years color fundus photographs of the right (L) and left (R) eyes. Note that the drusen patterns of each eye have changed. In the right eye, the drusen appear to have regressed further, sparing the center of the macula. The left eye shows enlargement of drusen as well as atrophy of the retinal pigment epithelium, especially involving the macula. Figure 3. Baseline plus 8 years color fundus photographs of the right (L) and left (R) eyes. Note that there is considerable maturation of the drusen pattern of the right eye accompanied by retinal pigment atrophy with sparing of the macula. The left eye shows considerable involvement of the center of the macula with drusen and retinal pigment epithelial atrophy.
(Images courtesy of Leo Semes, OD FAAO.)
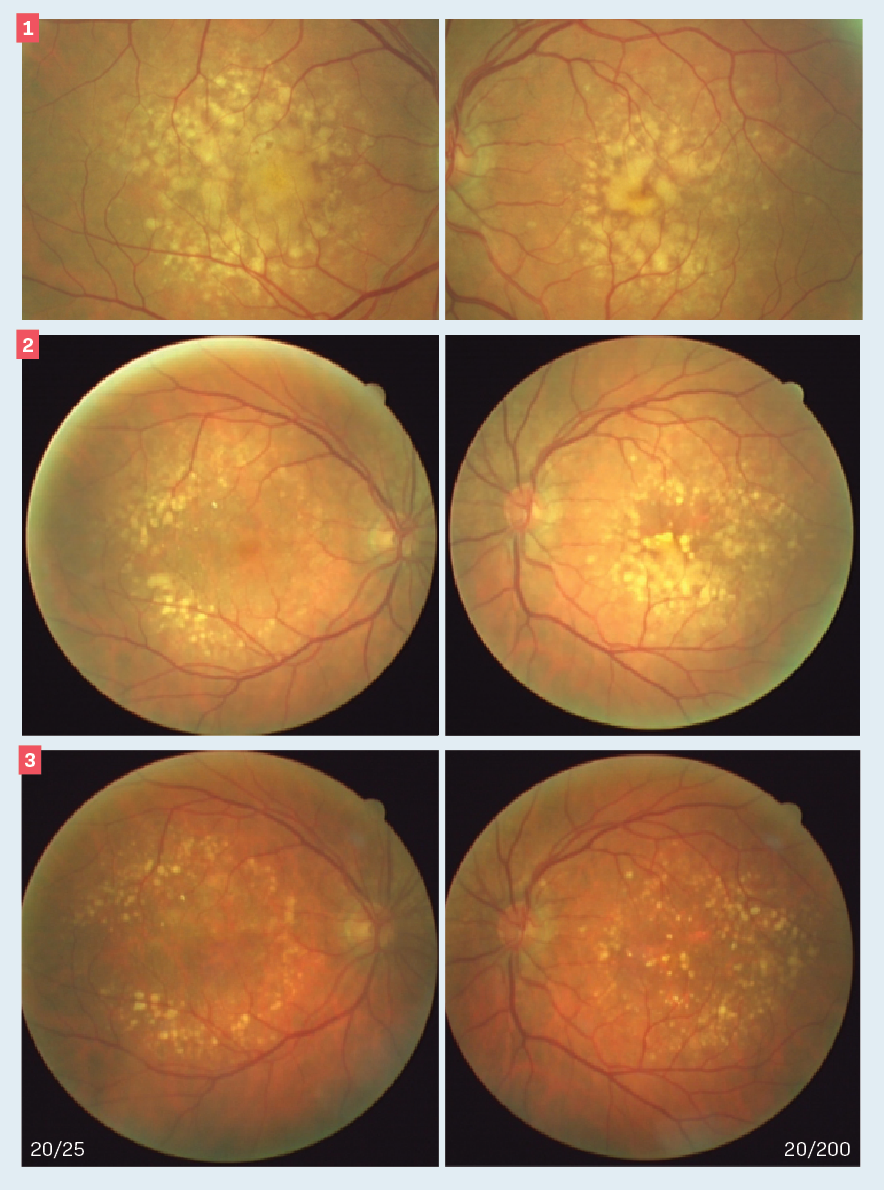
A 75-year-old patient was treated for dry age-related macular degeneration (AMD) over several years. His medical history is significant for treatment of systemic hypertension for approximately the past 15 years. At his initial presentation, visual acuity was 20/25 and 20/40 in the right and left eyes, respectively, despite the clinical appearance (Figure 1).
The diagnosis was nonneovascular AMD. According to the Beckman classification, this is category 3 and places the patient at high risk for advanced AMD.1 A recommendation for Age-Related Eye Disease Study (AREDS) formula supplementation was made, and the patient was asked to return for reevaluation in 6 months.
The patient was followed over the next 8 years (Figures 2, 3). Visual acuity decreased to 20/200 in the left eye but remained stable at 20/25 over this interval. The visual performance preservation in the right eye was due to a preserved island of central macular function.
Although there is no FDA-approved treatment for dry AMD, slowing progression utilizing the AREDS formula was the management option of choice in this case. Over the period of observation, the patient never developed a neovascular complication.
Discussion
Since the introduction and application of anti-VEGF agents, much of the focus over the past 15 years has been on treatment of neovascular AMD.2 Treatments for nonneovascular, or dry AMD, have been overlooked beyond lifestyle and dietary modifications, largely due the absence of effective surgical interventions. This is the state of affairs, despite the predominance of the dry form of AMD.3 What has been suggested is that although dry and wet forms of AMD have been thought of as being on a continuum, they should be recognized as separate disease entities.4
In the present case, there were few options. Genetic testing was performed to determine which formula would be the most appropriate. Recent imaging studies have focused on classification, baseline features, and trajectory for dry AMD, which is variable.5-8 Emerging research has involved targeting the complement system therapeutically.9 In addition, trials have been initiated on intravitreal injections for dry AMD, including antibody fragment technology, several of which are nearing FDA approval.10,11 This is significant as there may now be hope for preservation of visual performance and improvement, as seen with the anti-VEGF agents for wet AMD.
References
1. Ferris FL 3rd, Wilkinson CP, Bird A, et al; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. doi:10.1016/j.ophtha.2012.10.036
2. CATT Research Group; Martin DF, Maguire MG, Ying G, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. doi:10.1056/NEJMoa1102673
3. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606-2617. doi:10.1056/NEJMra0801537
4. Coleman HR, Chan CC, Ferris FL 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835-1845. doi:10.1016/S0140-6736(08)61759-6
5. Lindblad AS, Lloyd PC, Clemons TE, et al; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-1174. doi:10.1001/archophthalmol.2009.198
6. Keenan TD, Agrón E, Domalpally A, et al; AREDS2 Research Group. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125(12):1913-1928. doi:10.1016/j.ophtha.2018.05.028
7. Klein ML, Ferris FL 3rd, Armstrong J, et al; AREDS Research Group. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026-1031. doi:10.1016/j.ophtha.2007.08.030
8. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al; FAM-Study Group. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-472. doi:10.1016/j.ajo.2006.11.041
9. Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186-195. doi:10.1016/j.ophtha.2019.07.011
10. Emerging treatments bring hope for patients with geographic atrophy. Healio. Updated November 18, 2021. Accessed December 23, 2021. https://www.healio.com/news/ophthalmology/20211110/emerging-treatments-bring-hope-for-patients-with-geographic-atrophy
11. Khanani AM, Hershberger VS, Pieramici DJ, et al; Phase I Investigators. Phase 1 study of the anti-HtrA1 antibody-binding fragment FHTR2163 in geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2021;232:49-57. doi:10.1016/j.ajo.2021.06.017

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.