Early CAM therapy kick-starts healing
Combination therapy may reduce adverse events related to corticosteroids alone.
By facilitating corneal healing early in the disease process, patients could experience a lower disease burden, avoiding complications from improper healing, and have better visual outcomes. (Adobe Stock / rh2010)

Due to their established anti-inflammatory effects, corticosteroids are a mainstay in treating a wide variety of posterior and anterior segment conditions. Common complications with long-term steroid use, however, are adverse effects such as increased intraocular pressure and risk of cataract formation.1 Long-term steroid use also can delay corneal wound healing, hindering rapid recovery from uncomfortable conditions like infective epithelial keratitis.
To limit the risk of topical steroid-related adverse effects, adjunctive therapies can be used alongside reduced topical steroid regimens, limiting tissue exposure to steroids. Cryopreserved amniotic membrane (CAM) is one such adjunctive therapy, promoting healing by releasing anti-inflammatory, pro-reepithelization molecules onto the corneal surface.2
Vital to CAM’s healing properties is the heavy chain hyaluronic/pentraxin 3 (HCHA/PTX3) protein complex, which is known to reduce inflammation and scarring while increasing reepithelization, thereby accelerating healing.3,4 Amniotic membrane also can be used in a dehydrated form, but dehydrated amniotic membrane contains very little of the HC-HA/PTX3 complex, drastically reducing its benefits to the ocular surface.3,4
The speed of care and wound healing with CAM has revolutionized how quickly patients can get back to their daily lives. I call it my treatment over the weekend for ocular surface dryness. With CAM, I can get the front surface of the eye epithelized in just a couple of days.5 Nothing else on the market has that capability.
CAM is compatible with any therapy that my patients with corneal disease might use. With CAM, I do not have to stop my patients from taking their other essential treatments, like glaucoma medications or anti-infective agents.6 In fact, CAM has some antimicrobial properties, so I feel safe using it to accelerate healing alongside anti-infective agents.2
Additionally, CAM is a great option for patients who have difficulty adhering to treatment plans. Because CAM is applied in the office and lasts several days, patients who have trouble with drops regimens, due to either memory loss or reduced manual dexterity, are not at risk for undertreatment. On top of the treatment benefits, I find CAM an excellent option because it is simple and convenient to use, and does not require any extra equipment purchase to implement in office—easy to integrate into any practice.
Treatment goals and clinical pearls for earlier intervention
For any patient experiencing corneal disease, my goal is to return the cornea as close to homeostasis as possible. I want to see signs of reepithelization and promote healthy nerve tissue interaction with the functional tear unit. As an ocular surface–focused optometrist, I commonly see that, if the eye does not heal properly, patients develop dry eye signs and symptoms later, often because of reduced corneal nerve sensitivity and communication with nerves of the lacrimal glands, meibomian glands, and sometimes the entire functional tear unit.7 One consequence of this decreased communication is the loss of the regular blink reflex, which can further dry out the ocular surface.7 Even after initial treatment, chronic dry eye symptoms can occur and recur, particularly when the proper corneal healing and sensitivity were not effectively achieved.
For patients with neurotrophic keratitis (NK), cenegermin-bkbj (Oxervate; Dompé) topical drops can be an excellent option, as they contain a human nerve growth factor that supports corneal reinnervation.8 For my patients with NK, I find that initial placement of CAM, such as Prokera (BioTissue), followed by cenegermin-bkbj drops helps heal the tissue. I will then follow the drops course with another CAM to help protect the newly grown tissue. I call it the Prokera-Oxervate sandwich: Prokera/cenegermin-bkbj/Prokera. For patients who do not use cenegermin-bkbj, I often use CAM alone or with other therapies.
Previously, I thought CAM was more an option of last resort, reserved only for patients who had failed other therapies. But that is not the case; patients earlier in their disease course can benefit from CAM. It is often easier to treat a patient earlier than one who has experienced multiple failed treatments and years of tissue harm. Earlier intervention with CAM can also prevent current damage from worsening.
In deciding when to intervene with CAM, I first consider whether there is epithelial damage. Patients with superficial punctate keratitis, for instance, already have epithelial defects indicative of corneal damage and need corneal healing. I will start administering CAM with any eye that has punctate epithelial keratitis of at least 2+ because there is no reason to wait. If I wait and allow such corneal defects to progress, the patient can develop more advanced nerve and corneal damage, which is harder to treat. The eye may never recover from more advanced damage. CAM has the potential to regenerate corneal nerves, reepithelize, reduce inflammation, and help heal the cornea more effectively than with either no amniotic membrane or a dehydrated amniotic membrane.2,3
In most cases, I use Prokera in conjunction with my first-line therapy options, such as topical antibiotics or corticosteroids. There are no contraindications to using other oral or topical therapies with CAM.6 Once CAM is applied, the corneal surface begins to heal. Faster healing translates to the patient experiencing quicker relief from ocular surface disease and injury. As I treat corneal tissue with other medications, such as anti-infectives, CAM can also function as a barrier, protecting newly healed tissue. CAM is especially helpful for patients on topical glaucoma medications, which commonly contain benzalkonium chloride, a preservative known to degrade corneal tissue over time.9
When communicating with patients about their corneal disease and CAM, I like to use corneal staining photography. Digital images of the stained cornea can help patients visualize their corneal disease and help physicians describe how CAM can optimize healing.
I often use the analogy that CAM is like fertilizer and will kick-start their healing process. I also let patients know that they are going to have some sensation and feel something in their eye for a few days but that they can use nonpreserved artificial tears if they like. CAM is robust, so a patient will not dissolve the membrane with nonpreserved artificial tears or other concurrent medications. For most patients, I gently tape their eyelid closed (tape tarsorrhaphy) to help reduce foreign body sensation. Patient instructions are often about setting proper expectations. When patients know what to expect with CAM, there are few complaints; I have never had a patient decline treatment when the CAM treatment process is explained well.
CAM in practice: A case study in ocular rosacea
Recently, I treated a woman aged 45 years whose chief concern was chronic ocular redness and irritation. Past treatments with hot compresses and artificial tears failed to improve her symptoms. Due to moderate facial and severe eyelid telangiectasia, the diagnosis was ocular rosacea. With sodium fluorescein staining, I saw that she had diffuse corneal punctate staining with some coalescing (Figure 1A).
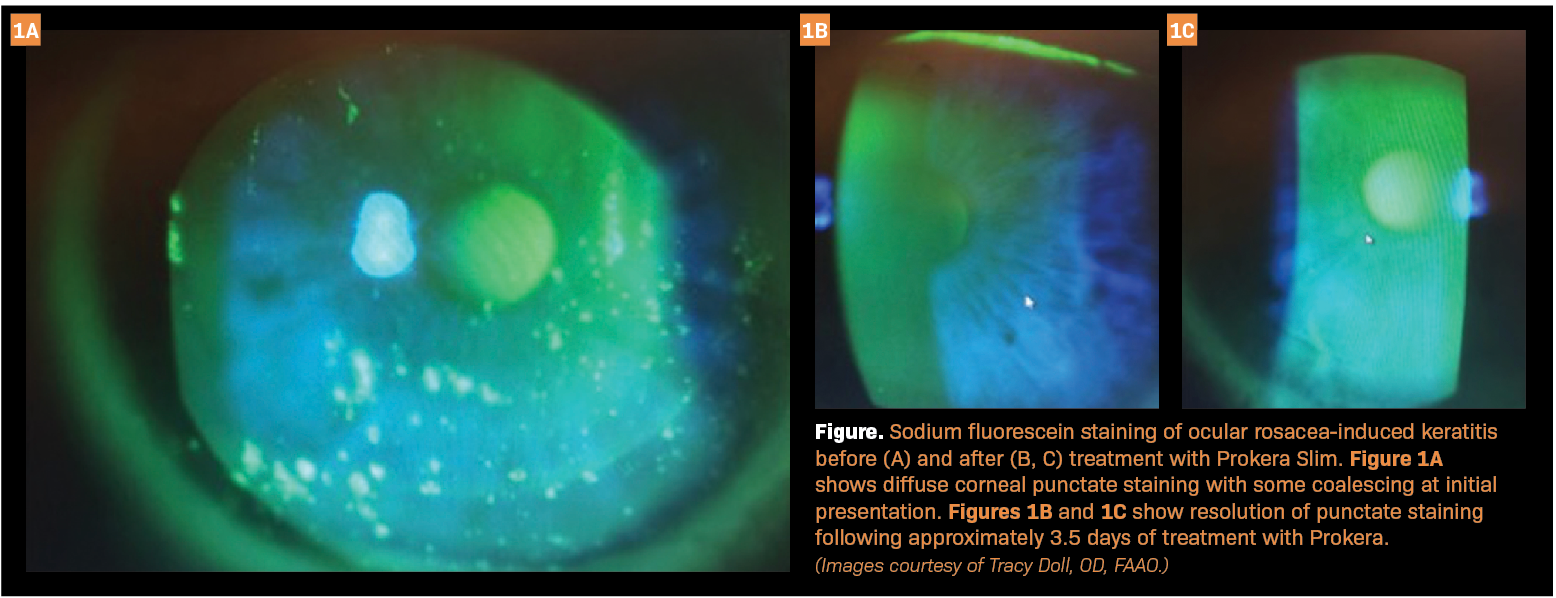
I also did a cotton-wisp test and found that she had reduced bilateral corneal sensitivity. I placed a Prokera Slim on her eyes on a Friday and removed it the following Monday, a treatment duration of approximately 3.5 days. After removing Prokera, I saw that she had complete resolution of the punctate staining (Figures 1B and 1C), and she reported that her symptoms were improved as well. To treat the underlying rosacea, she went on to receive intense pulsed light therapy. Over the following 3 years, the punctate staining did not recur. In this case, using Prokera highlights the speed of tissue healing and symptom resolution when used alongside other ocular surface disease therapies.
Regenerative medicine in the future
Patients are becoming increasingly familiar with regenerative medicine, especially in the Pacific Northwest, where I practice, and I have seen many positive patient outcomes after using CAM. I want to emphasize earlier intervention with CAM because the modern definition of ocular surface dryness includes neurosensory abnormalities,10 which can stem from damage to corneal nerves through corneal epithelial disease and lead to more tissue damage. By facilitating corneal healing early in the disease process, patients could experience a lower disease burden, avoiding complications from improper healing, and have better visual outcomes.
References
1. Gaballa SA, Kompella UB, Elgarhy O, et al. Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. 2021;11(3):866-893. doi:10.1007/s13346-020-00843-z
2. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18(2):193-204. doi:10.1007/s10561-017-9618-5
3 .Mead OG, Tighe S, Tseng SCG. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10(1):13-21. doi:10.4103/tjo.tjo_5_20
4. Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SC. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23(10):465-474, 476. doi:10.12968/jowc.2014.23.10.465
5. John T, Tighe S, Sheha H, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:6404918. doi:10.1155/2017/6404918
6. Prokera. Prescribing information. BioTissue; 2023. Accessed August 22, 2023. https://biotissue.com/wp-content/uploads/sites/3/2019/09/prokera-insert_PI-BT-003E_V3.pdf
7. Matossian C, Crowley M, Periman L, Sorkin S. Personalized management of dry eye disease: beyond artificial tears. Clin Ophthalmol. 2022;16:3911-3918. doi:10.2147/OPTH.S384819
8. Oxervate. Prescribing information. Dompé; 2019. Accessed August 22, 2023. https://oxervate.com/wp-content/uploads/2022/06/OXERVATE_Prescribing_Information_102019.pdf
9. Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye (Lond). 2022;36(2):361-368. doi:10.1038/s41433-021-01668-x
10. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812. doi:10.1016/j.jtos.2017.08.003
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.