Know the ocular effects of eyelash growth serums
Creating longer lashes may lead to clinical concerns
Figure 1. Lash elongation exceeding one-third horizontal eye width.


The eyes are one of the biggest markers for facial attractiveness and overall beauty.1,2 The quest for longer, thicker, and darker eyelashes propelled both the pharmaceutical and beauty industries into action. The United States share of the market, which is currently the largest, is expected to reach $234.59 million by 2023.1 Growing eyelashes is big business.
From IOP to lashes
Bimatoprost (Lumigan 0.03%, Allergan) was first approved by the U.S. Food & Drug Administration (FDA) for the treatment of glaucoma or ocular hypertension in 2001 as an intraocular pressure (IOP)-lowering agent.3 As was noted in the clinical trials, glaucoma/ocular hypertension patients using this topical eyedrop could experience the side effect of darker and thicker eyelash growth.
Bimatoprost (and other prostaglandin analogs) target the anagen phase of the eyelash growth cycle, resulting in longer, thicker eyelashes with more melanin deposition. Prostaglandin analogs may also increase the number of eyelashes in the lash follicle itself.
In 2008, bimatoprost was re-launched as Latisse (Allegan), an FDA-approved therapy for the treatment of hypotrichiasis.3 It is commonly used to treat trichotillomania, chemotherapy-induced eyelash loss, and alopecia areata lash loss. Nightly application of Latisse, applied in a thin line along the eyelash margins, showed maximal effectivity of lash enhancement at 16 weeks of usage.3
In its first year on the market, Latisse was able to pull in $47.7 million, making it one of the most successful pharmaceutical launches to date.5
OTC growth serums
The beauty industry responded to the success of Latisse by rolling out its own versions of over-the-counter eyelash growth serums (OTC-ELGS). Patients might not know that OTC-ELGS can contain synthetic prostaglandins with the same side effects as the prescription version.
Unlike pharmaceutical companies, cosmetic companies are not required to list potential side effects of a prostaglandin analog (or any ingredient) on their packaging.6
Known side effects of prostaglandin analogs (organic or synthetic) include:4
Conjunctival hyperemia
Skin or iris pigmentation
Pruritus (itching)
Lash loss/fall
Lowered IOP
Topical prostaglandin analog eyedrops have also been identified as a potential causative factor for meibomian gland dysfunction and varying degrees of ophthalmopathy, including thinning of eyelid margins and acquired blepharophimosis (decrease in palpebral aperture).7,8 It should be noted that there is no known percentage of prostaglandin analog that is “side-effect free.”
A recent survey of 154 current and past OTC-ELGS users showed that 40 percent of individuals who tried this type of cosmetic dropped-out of OTC-ELGS use. The top reason for discontinuation/dropout was “side effects” (noted by 43 percent of dropouts), followed by cost (27 pecent of dropouts).9
Serum ingredients
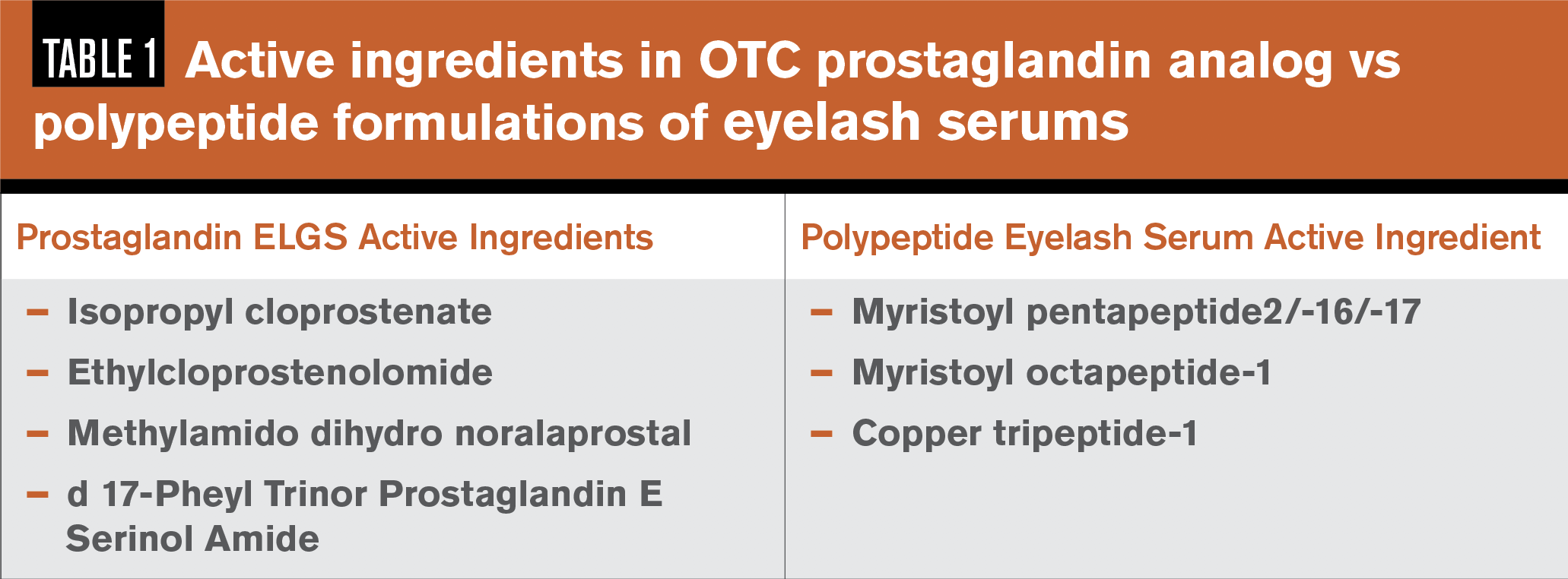
A synthetic prostaglandin can be difficult to spot as an active ingredient in an OTC-ELGS because cosmetic packaging does not always list that one is included. The key is to look for “prost” as an indicator of a potential synthetic prostaglandin ingredient. Isopropyl Cloprostenate is one of the most common OTC-ELGS active ingredients, with other prostaglandin analog agents in OTC-ELGS listed in Table 1.10
Some cosmetic companies have chosen to re-formulate their serums following the release of a report in 2013 by the Swedish Medical Products Agency, exposing cosmetic companies using synthetic prostaglandin analogs in their OTC-ELGS and banning their use.10
There are lash serums that do not contain synthetic prostaglandins. These alternative formulations include polypeptide and lipopeptide preparations of amino acids that support growth in the follicle, acting in more of a “conditioning” manner.11,12
Common lipopeptide ingredients are listed in Table 1.13,14 Even the lipopeptide and polypeptide versions do not necessarily come without risk and may contain other irritating ingredients, so it is important to review the ingredient list.
The cosmetic industry is largely unregulated in the United States, with only 11 ingredients prohibited for use, due to danger to health. This is a tiny fraction of the amount of chemicals that are banned for use in cosmetics in Europe, which lists 1328 banned ingredients.15,16
Ocular irritation test
Ocular irritation potential in cosmetics is rated using the Draize eye irritancy test, created in 1944, in-vivo on albino rabbits. Cosmetics would be deemed “irritating” if redness, swelling, or cloudiness in the rabbit eye is noted.17
A newer test, the EpiOcular eye irritation test, has been created in pursuit of an alternative to animal testing. The EpiOculareye irritation test is able to detect mild to moderate irritation using a three-dimensional, in-vitro tissue model of the human corneal epithelium.18-20
What is interesting about this model is that a chemical is classified a “non-irritant (GHS unclassified),” if the viability of the tested tissue is >60 pecent.20 The other 40 percent of the cells do not have to survive. Neither the Draize or EpiOcular eye irritation tests are required for cosmetics to hit the market in the United States.
The best alternative is for eyecare providers and patients alike to use ingredient-checking websites and phone apps to review cosmetic ingredients (see Table 2).

Prescription bimatoprost can be prescribed via telehealth through a variety of websites, including Rory.com, Apostrophe.com, and SkinSolutionsMD.com. The video telehealth consultation consists of case history questions with an MD, but no external examination with biomicroscopy is required. Patients will receive prescription medications with package inserts and education regarding potential side effects.
In that recent survey of 154 OTC-ELGS current and past users, respondents indicated that the most common methods of obtaining and OTC-ELGS were online (37 respondents) and via a consultant (31 respondents). Only 7 survey-takers indicated they purchased OTC-ELGS from a doctor’s office.9
The eyecare provider may not know about patient use of ELGS. This cosmetic is clear, unlike other eye cosmetics including mascara, eyeshadow, or eyeliner. Methods of detection are case history questions (do you use an eyelash growth serum), and physical examination of eyelash length.
The healthiest eyelash length is one-third the horizontal eye width, canthus to canthus. Lengths that exceed this ratio can result in the funneling of air and debris to the ocular surface.20Eyelashes that seem “too long” could signal that a patient is using an ELGS (Figure 1).
Click here to take the quiz that accompanies this article
Conclusion
With the potential for side effects and the “wind-tunnel” effect, patients with chronic ocular inflammatory conditions, including ocular surface dryness, should avoid prostaglandin ELGS. With 44 percent of women experiencing negative emotions without wearing makeup,21 even these patients may be unwilling to give up prostaglandin ELGS use.
Eyecare providers may need to go the “extra length” with a healthy recommendation for a reasonable switch. The healthier alternative is a polypeptide formulated lash serum conditioner with safer ingredients, as vetted by a cleaner makeup app (Table 2). Should a patient opt to continue using a prostaglandin ELGS, this individual should be followed regularly to monitor ocular health and educated and re-educated on common side effects.

References
1. Strauch B, Lang A, Ferder M, Keyes-Ford M, Freeman K, Newstein D. The ten test. Plast Reconstr Surg. 1997 Apr;99(4):1074-8.
2. Boffano P, Roccia F, Gallesio C, Karagozoglu KH, Forouzanfar T. Diplopia and orbital wall fractures. J Craniofac Surg. 2014;25(2):e183-5.
3.Park MS, Kim YJ, Kim H, Nam SH, Choi YW. Prevalence of Diplopia and Extraocular Movement Limitation according to the Location of Isolated Pure Blowout Fractures. Arch Plast Surg. 2012 May;39(3):204-8.
4. Burm JS, Chung CH, Oh SJ. Pure orbital blowout fracture: new concepts and importance of medial orbital blowout fracture. Plast Reconstr Surg. 1999 Jun;103(7):1839-49.
5. Schönegg D, Wagner M, Schumann P, Essig H, Seifert B, Rücker M, Gander T. Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J Craniomaxillofac Surg. 2018 Sep;46(9):1544-1549.
6. Putterman AM. Management of orbital floor blowout fractures. Aesthet Surg J. 2018 Apr 6;38(5):488-490.
7.Freund M, S. Hähnel S, Sartor K. The value of magnetic resonance imaging in the diagnosis of orbital floor fractures. Eur Radiol. 2002 May;12(5):1127-33.
8.Roelofs KA, Starks V, Yoon MK. Orbital Emphysema: A Case Report and Comprehensive Review of the Literature. Ophthalmic Plast Reconstr Surg. Jan/Feb 2019;35(1):1-6.
9. Reiss B, Rajjoub L, Mansour T, Chen T, Mumtaz A. Antibiotic Prophylaxis in Orbital Fractures. Open Ophthalmol J. 2017 Jan 31;11:11-16.
10. Ben Simon GJ, Bush S, Selva D, McNab AA. Orbital cellulitis: a rare complication after orbital blowout fracture. Ophthalmology. 2005 Nov;112(11):2030-4.
11. Wang JJ, Koterwas JM, Bedrossian EH Jr, Foster WJ. Practice patterns in the use of prophylactic antibiotics following nonoperative orbital fractures. Clin Ophthalmol. 2016 Oct 27;10:2129-2133.
12. Gheza C, Bravo-Soto G, and Varas G. Are postoperative prophylactic antibiotics effective for orbital fracture? Medwave. 2018 Jul 30;18(4):e7234.
13. Lee S H, Lew H, Yun YS. Ocular motility disturbances in orbital wall fracture patients. Yonsei Med J. 2005 Jun 30;46(3):359-67.
14. Caranci F, Cicala D, Cappabianca S, Briganti F, Brunese L, Fonio P. Orbital fractures: role of imaging. Semin Ultrasound CT MR. 2012 Oct;33(5):385-91.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.