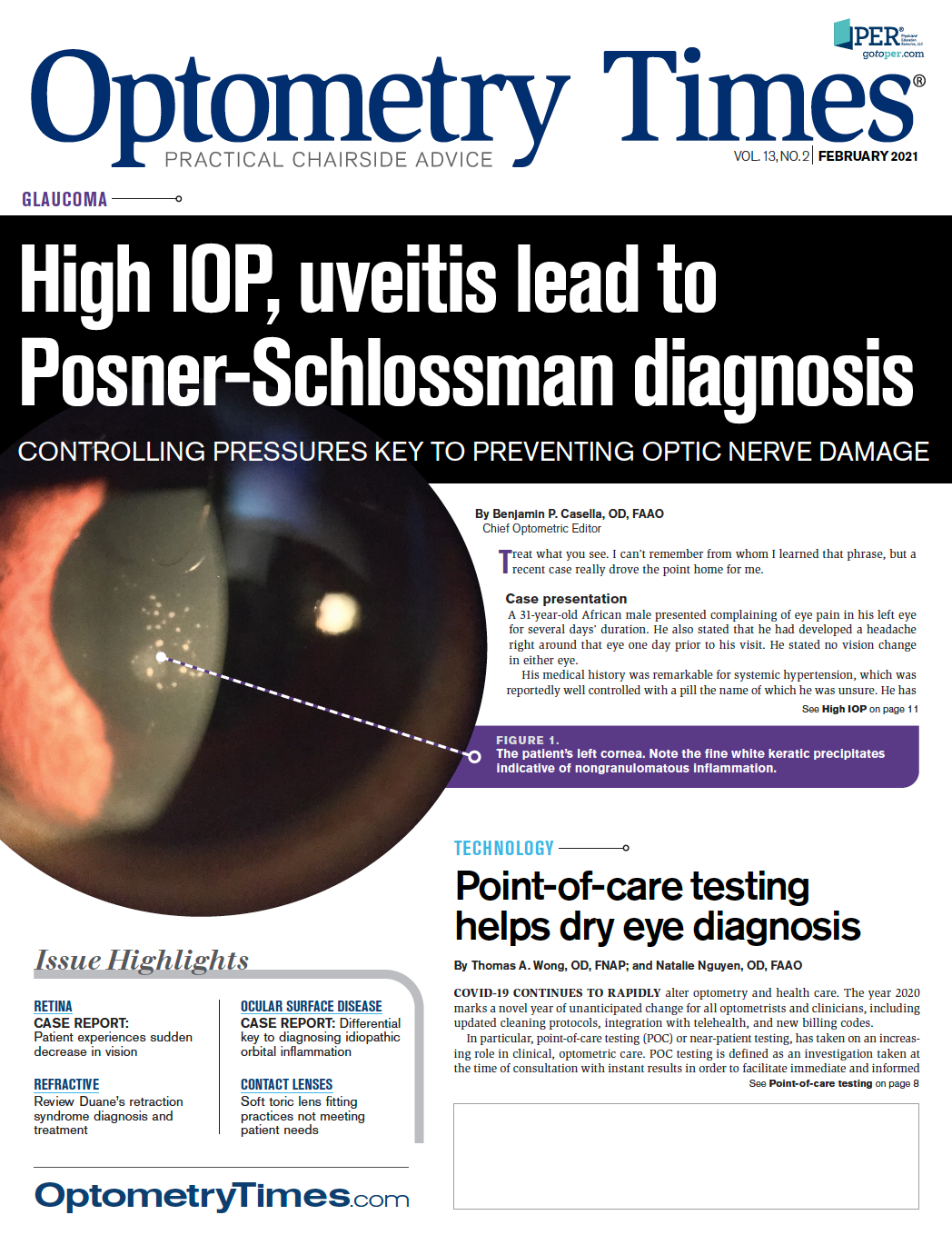
Point-of-care testing helps dry eye diagnosis
Due to the COVID-19 pandemic, optometric care is experiencing a rapid shift towards medical diagnostic testing that is taken at the time and place of patient care.


COVID-19 CONTINUES TO RAPIDLY alter optometry and health care. The year 2020 marks a novel year of unanticipated change for all optometrists and clinicians, including updated cleaning protocols, integration with telehealth, and new billing codes.
In particular, point-of-care testing (POC) or near-patient testing, has taken on an increasing role in clinical, optometric care. POC testing is defined as an investigation taken at the time of consultation with instant results in order to facilitate immediate and informed
decisions regarding patient care.1
Testing, results, and diagnosis performed at the slit lamp and in the exam room where optometrists come in close contact with patients is often supplemented by new technologies like POC testing.
In today’s socially distant world, it is important to minimize time spent in close proximity without compromising patient care and outcomes. An advantage is the ability to effectively capitalize on technological advancements to maximize POC testing, particularly with dry eye, ocular surface disease, and conjunctivitis.
The eye’s anterior segment begins at the tear film, the primary refractive surface of the eye. The tear film can be compromised by lack of tear production (aqueous deficient) or too-rapid disappearance of the tears (evaporative).2 An example of eye care POC testing is TearLab’s Osmolarity System which allows for objective and quantitative measurement of tear osmolarity, a biomarker of dry eye. Tear osmolarity has been found to be an important metric in both the diagnosis and classification of dry eye disease.3
Schirmer test
A long-standing method of quantifying tear production is the Schirmer test. Schirmer 1 measures total tear production including basal and reflex, while Schirmer 2 measures reflex secretion exclusively and involves nasal stimulation following strip insertion into the lower fornix.
Generally, Schirmer 1 is performed more frequently in the optometric setting with a normal reading of >15 mm per eye.4 From a POC perspective, this allows an optometrist to make an informed decision on first-line treatment regimens such as artificial tears versus punctal plugs in the management of aqueous deficient and evaporative dry eye. POC testing brings real-time diagnostic decision making closer to the patient. Furthermore, easy repeatability of diagnostic testing increases both accuracy and precision, thus improving patient outcomes.
Tear film break-up time
Another great example of POC testing for dry eye is tear film break-up time (TFBUT).5 Optometrists have the advantage of being able to evaluate TFBUT while at the slit lamp prior to measuring intraocular pressure (IOP), e.g. performing Goldmann applanation tonometry without significantly increasing overall exam time and minimizing provider and patient risk of exposure (especially important in a COVID-19 world).
A critical test in assessing the integrity of the tear film and cornea, TFBUT allows optometrists to be able to both provide POC testing without the requirement of additional resources or equipment necessary to test/quantify, receive results, diagnose, or assess the progression of evaporative dry eye.
MMP-9 testing
Another valuable resource is InflammaDry (Quidel), the first and only in-office test that detects elevated levels of matrix metalloproteinase-9 (MMP- 9).6 It has been shown that MMP-9 is an inflammatory marker that is consistently elevated in the tears of patients with dry eye disease.7,8 Evaluation of MMP-9 levels in patients has the potential to target an underlying inflammatory mechanism of dry eye that would otherwise go undetected.
InflammaDry is capable of assisting a clinician’s ability to test, diagnose, and treat inflammatory-related dry eye while simultaneously minimizing additional time required in the exam room. With results available in 10 minutes, InflammaDry has great potential to provide an individualized treatment plan for the patient from initial examination
Several studies have evaluated the sensitivity and specificity of InflammaDry in the diagnosis of dry eye disease using various metrics and inclusion criteria, such as Schirmer testing, keratoconjunctival staining, ocular surface disease index (OSDI), and TFBUT. One study found InflammaDry to have a sensitivity of 85% (121 of 143 patients) and specificity of 94% (59 of 63 subjects) in the evaluation of dry eye disease.9 To better understand the utility of InflammaDry in the evaluation of dry eye, further studies are needed. MMP-9 is known to be elevated in conjunctivochalasis, vernal keratoconjunctivitis, and contact lens wear. The multifactorial nature of dry eye disease necessitates that optometrists analyze how to best utilize InflammaDry in their diagnostic regimen.10
Conjunctivitis
Another important application of POC testing is in diagnosing and managing acute conjunctivitis— especially adenovirus conjunctivitis which affects 1 in 4 patients with acute conjunctivitis.17,18 AdenoPlus (Quidel) has high specificity for diagnosing adenoviral conjunctivitis (92%), but a lower sensitivity (50%), demonstrating the importance of real-time polymerase chain reaction (PCR) confirmation.19
During the COVID-19 pandemic, clinical improvement amendment waivers have been granted by the U.S. Food & Drug Administration (FDA). QuickVue Adenoviral conjunctivitis Clinical Laboratory Improvement Amendments (CLIA)- waived testing allows for rapid, accurate, in-office, diagnostic decision making.
Differential diagnosis based on objective clinical findings (lid edema, serous discharge, hyperemia, conjunctival follicles, pre-auricular nodes) can provide accurate diagnosis of adenovirus conjunctivitis However, the use of POC testing has been shown to further improve diagnostic accuracy.21 “Area under the curve” (AUC) data was used to compare the prediction performance of different diagnostic models.
When Quickvue was added to slit lamp and physical exam findings, the AUC predictive values increased from 0.84 to 0.95. A more accurate, real-time diagnosis of adenoviral conjunctivitis can prevent the spread of infection, reduce potential ocular allergies and toxicities to unnecessary antibiotic use, and help prevent antibiotic resistance by reducing unnecessary antibiotic prescriptions.20
Conclusion
Health care continues to evolve at a rapid rate, especially in the setting of COVID-19. This secondarily has induced a shift in optometric care toward POC testing, which has shown to be pertinent in the treatment and management of ocular surface disease and adenoviral conjunctivitis.
With the addition of POC testing to traditional slit lamp and physical examination findings, especially in an inter-professional setting, the optometrist is able to improve eyecare diagnosis and management while maintaining the safety of patients, staff, and clinicians.

References
1. Price CP. Point of care testing. BMJ. 2001 May 26;322(7297):1285-1288.
2. TFOS DEWS II Patient Summary. Available at: https:// www.tearfilm.org/dettnews-tfos_dews_ii_patient_ summary/6814_5519/eng/. Accessed 12/8/20.
3. Lemp MA, Bron AJ, Baudouin C, Benítez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-798.e1.
4. Senchyna M, Wax MB. Quantitative assessment of tear production: A review of methods and utility in dry eye drug discovery. J Ocul Biol Dis Infor. 2008 Mar;1(1):1-6.
5. Welch D, Ousler GW, Abelson MB. An approach to a more standardized method of evaluating tear film break-up time. Invest Ophthalmol Vis Sci.2003 May;44(13):2485.
6. Bethke W. Putting dry eye to the test. Rev Ophthalmol. Available at: https://www.reviewofophthalmology.com/article/ putting-dry-eye-to-the-test. Accessed 12/8/20.
7. VanDerMeid KR, Su SP, Krenzer KL, Ward KW, Zhang JZ. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011 Apr 27;17:1056-63.
8. Chotikavanich S, de Paiva CS, Li de Quan, Chen JJ, Bian F, Farley WJ, Pflugfelder SC. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3203-9.
9. Sambursky R, Davitt WF 3rd, Latkany R, Tauber S, Starr C, Friedberg M, Dirks MS, McDonald M. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013 Jan;131(1):24-8.
10. Lanza NL, Valenzuela F, Perez VL, Galor A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul Surf. 2016 Apr;14(2):189-195.
11. Hill G. Offer IPL as a treatment for MGD and dry eye disease. Optometry Times. Available at: https://www. optometrytimes.com/view/offer-ipl-treatment-mgd-and-dry-eye-disease. Accessed 12/8/20.
12. Toyos R, Buffa C, Youngerman S. Case report: Dry eye symptoms improve with Intense Pulsed Light treatment. Toyos Clinic. Available at: https://toyosclinic.com/blog-feed/ jpst1fb36fd0roveb4jddg2hgveaf5. Accessed 12/8/20.
13. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. 2015 Jan;33(1):41-46.
14. Mayo Clinic. Intense pulsed light for treatment of dry eye disease. Available at: https://www.mayoclinic.org/medical-professionals/ophthalmology/news/intense-pulsed-light-for-treatment-of-dry-eye-disease/mac-20430229. Accessed: 12/8/20.
15. Kent C. Knowledge and Tech: Treating Dry Eye, 2019. Rev Ophthalmol. Available at: https://www.reviewofophthalmology. com/article/knowledge-and-tech-treating-dry-eye-2019. Accessed 12/8/20.
16. Dell SJ, Desai NR. IPL + thermal pulsation: A thorough approach to dry eye. Ophthalmol Times. Available at: https:// www.ophthalmologytimes.com/view/ipl-thermal-pulsation-thorough-approach-dry-eye. Accessed 12/8/20.
17. Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, McDonald M, DellaVecchia M, Luchs J. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013 Jan;131(1):17-22.
18. Sambursky R, Tauber S, Schirra F, Kozich K, Davidson R, Cohen EJ. The RPS adeno detector for diagnosing adenoviral conjunctivitis. Ophthalmology. 2006 Oct;113(10):1758-64.
19. Holtz KK, Townsend KR, Furst JW, Myers JF, Binnicker MJ, Quigg SM, Maxson JA, Epsy MJ. An Assessment of the AdenoPlus Point-of-Care Test for Diagnosing Adenoviral Conjunctivitis and Its Effect on Antibiotic Stewardship. Mayo Clin Proc Innov Qual Outcomes. 2017 Jul;1(2):170-175.
20. Shorter E, Perera C, Migneco M, Whiteside M, Harthan J, Hartwick A, Johnson S, Morettin C, Huecker J, Than T, Gordon M. Improving accuracy of adenoviral conjunctivitis diagnosis. Invest Ophthalmol Vis Sci.2020 June;61(7):4900.
21. Holtz K, Townsend K, Furst J, Myers J, Binnicker M, Quigg S, et al. An Assessment of the AdenoPlus Point-of-Care Test for Diagnosing Adenoviral Conjunctivitis and Its Effect on Antibiotic Stewardship. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 10.1016/j.mayocpiqo.2017.06.001
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.