Retinal considerations crucial for accurate biometry
Evaluating accuracy of biometry is paramount in eyes with history of retinal pathology

As ophthalmologic diagnostic testing advances in resolution, depth, and scale, we are increasingly able to qualify and quantify disease progression. Specifically, with optical coherence tomography (OCT), we moved from time domain (TD-OCT) imaging to spectral domain (SD-OCT), which was a great increase in sensitivity and clarity.
Currently, the most advanced technique uses a swept-source (SS-OCT) input to capture the SD-OCT data, resulting in even more accurate scanning. In parallel, technology for biometry—the general term for eye measuring for intraocular lens (IOLs)—has also made progress.
A key turning point was the switch from ultrasound measurement to optical biometry. This allowed for greatly increased accuracy in measuring the 2 most important biometric variables: axial length (AL) and keratometry. Combining the 2 advances ideally results in reliable and precise measurements for IOL calculation.
Paramount in collecting the data, analyzing the measurements collected by the state-of-the art machines is up to us as clinicians and drivers. As accurate as measurements can be—if they are computing, for example, an AL that measures from the cornea to the retina—pathology in the posterior pole can greatly devalue the results.
Here we will discuss AL/biometry basics for most accurate IOL calculation as well as crucial retinal pathology considerations when evaluating retinal health and its resounding effects: epiretinal membranes (ERMs), age-related macular degeneration (AMD), and staphylomas.
IOLs
AL (in mm) measurement is a crucial step in IOL power calculations. If the length errs by 0.1 mm, it equates to an approximately 0.27-diopter (D) error in the spectacle plane.1 Accuracy is a necessity. AL is measured from the anteriormost plane of the cornea through to either the retinal pigment epithelium (RPE) or inner limiting membrane (ILM), depending on which mode of biometry is utilized.2 Using optical biometry (like the IOLMaster [Zeiss]), AL measurement is obtained by noncontact partial coherence laser interferometry that is based on the signal of the RPE.3
Analyzing scans
In contrast, when using acoustic biometry (A-scan)—via either immersion or direct contact—AL measurement is obtained by A-scan ultrasound based on the signal of the ILM.3 Optical biometry is very precise and removes the variable of user error, because compression of the cornea is not required.
However, acoustic or contact biometry may be more reliable in cases where corneal opacity, dense cataract, patient inability to fixate, or other pathology does not allow for the waves to penetrate to the retina. Utilizing either method, it is vital to analyze the strength and reliability of the scan lest the technique measures to an incorrect point on the retina, removing the accuracy.
Focusing on retinal pathology, if the anatomy of the retina is raised, the AL could measure short—conversely, if the retina is atrophied or bowed posteriorly, the AL could measure long. Utilizing the most appropriate mode based on pathology is integral. Remain mindful of the posteriormost stopping point of the mode (ILM vs RPE).
ERM
ERM, also known as cellophane maculopathy, macular pucker, or simply scar tissue, is typically defined as an avascular, fibrocellular membrane attached over the inner surface of the retina or the ILM of the macula.4,5 There is often traction where the tissue is attached, thus creating elevation of the retina, visible on OCT as thickening of the central fovea.5
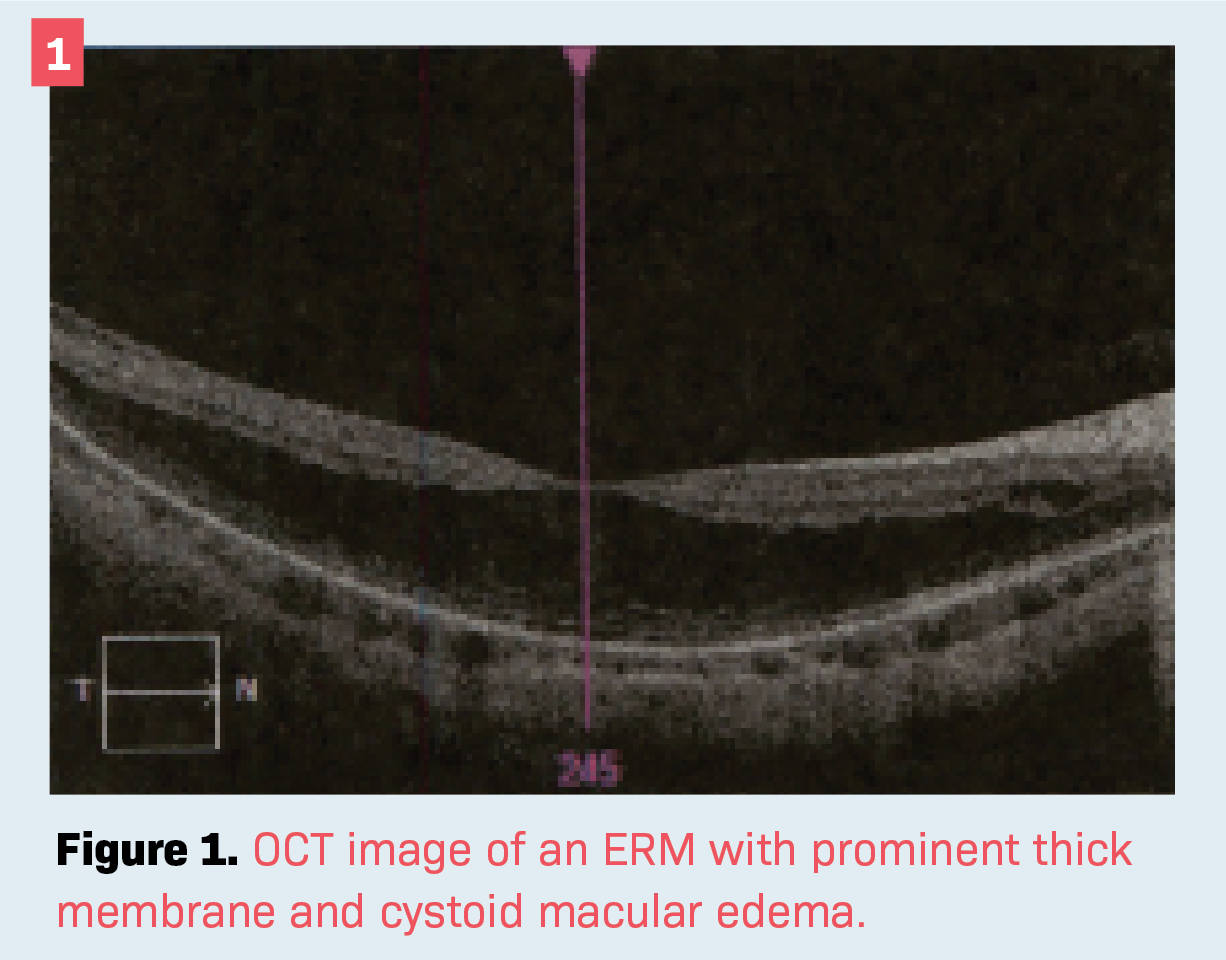
Often there may be such traction that cystoid spaces filled with fluid are visible. Figure 1 shows an OCT image of an ERM with prominent thick membrane and cystoid . Note the absence of a foveal depression. As pertains to biometry, when the ILM is elevated it may be falsely read as the posteriormost point on the retina, artificially shortening the AL. For example, if the ERM is 400 µm in height, it would subtract 0.4 mm in AL, which could skew the IOL measurements by approximately 1 D.
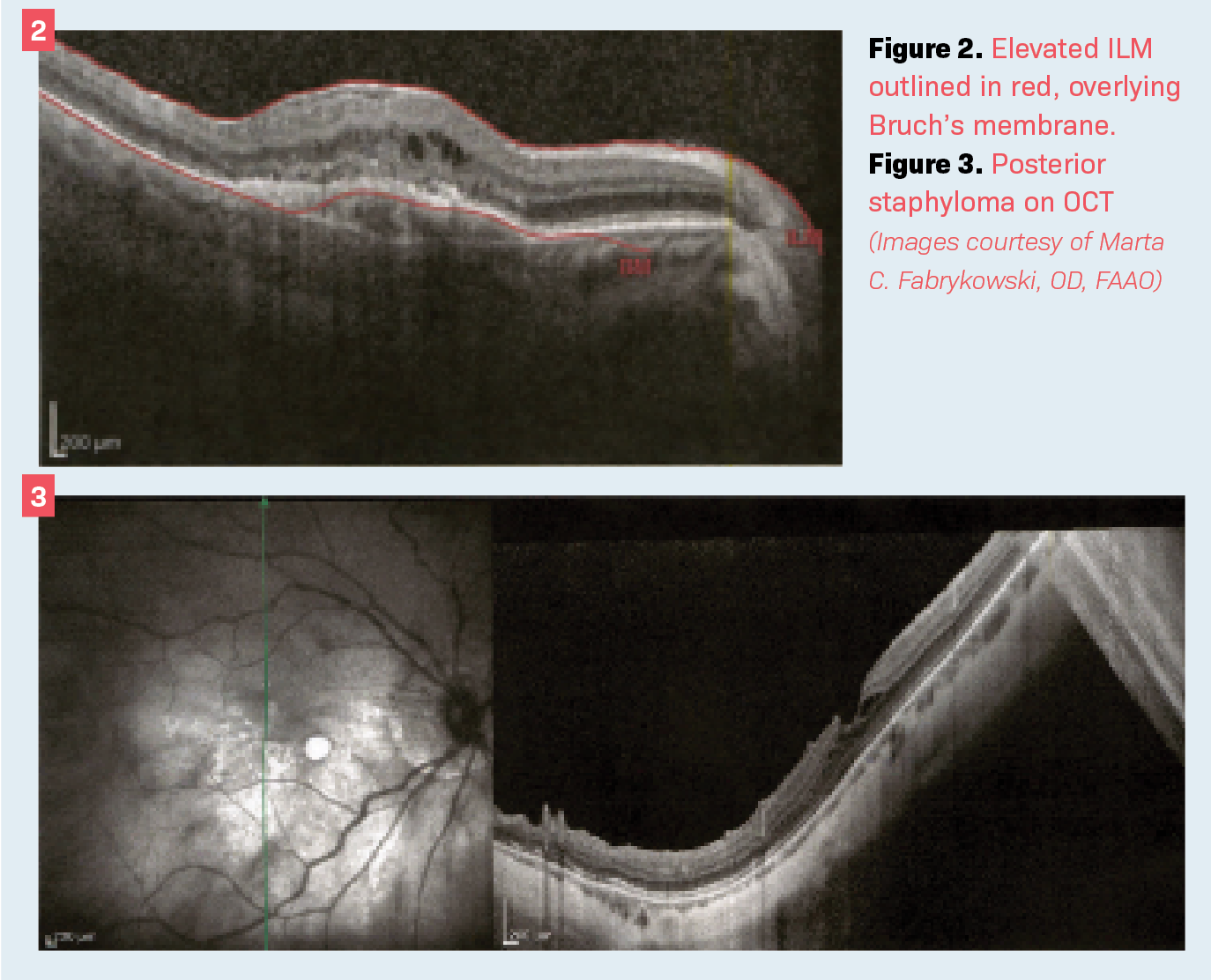
In Figure 2 the right eye has an ERM, shortening the AL by nearly 0.5 mm compared with the left. It is important to note that eyes may differ in length—but typically not more than 0.3 mm—and the wider the disparity, the more closely one should examine the scan.6
Related: A guide to the latest presbyopia-correcting IOLs
AMD
As is widely known, AMD occurs in wet and dry varieties. As pertains to biometry, they can cause both elevation and depression of the retina when considering the furthest point from the cornea.
In the case of wet AMD, much like cystoid spaces in ERMs, the retina is elevated and thus would measure closer to the cornea. See Figure 2 with elevated ILM outlined in red, overlying the Bruch membrane. This would cause the AL to falsely measure shorter than expected. In the case of dry AMD, the retina would atrophy.
There is no fluid; instead, chorioretinal thinning—sometimes referred to as geographic atrophy—could cause a false increase in AL as the retina moves farther from the cornea. Thus, in patients with AMD, there should be extra vigilance in examining which portion of the retina was measured to determine the AL.
Posterior staphyloma
A posterior staphyloma, seen as an outpouching of the retina via bulging of the sclera, is often found in highly myopic eyes (> –8D).7 Ironically, its prevalence increases with increasing AL, with the outpouching mostly affecting the sclera, choroid, and RPE.7
Figure 3 shows posterior staphyloma on OCT. Note that the thinning choroid and irregular scleral surface create an outpouching. It is easy to imagine the AL being potentially measured longer if it is determined through the staphyloma. Highly myopic patients often prefer to remain nearsighted after cataract surgery, so it becomes a bit less crucial to measure AL precisely. However, it is still important to keep in mind and analyze closely in longer eyes.
Conclusion
Evaluating the accuracy of biometry is paramount in eyes with known history of retinal pathology. Patients without such known history should be thoroughly evaluated to rule it out.
Paying close attention to the location of the biometry signal peaks, as well as comparing to the fellow eye, are excellent tactics for beginning evaluation. If in doubt, contrasting with previous years’ biometry may also shed light on accuracy, because AL should not change much with age.
Assessing the whole picture of refractive history as well as ocular pathology is ideal for maintaining the collection and utilization of AL integrity. If accurate evaluation of said eye is not possible (due to opacities or compromised ocular structure), it is useful to assess the AL of the fellow eye if the refractive and ocular health histories are similar.
Lastly, if confidence in AL or other components of biometry is low, Optiwave Refractive Analysis (ORA; Alcon) is a great option for estimating IOL power from AL. As technology to qualify and quantify pathology improves, we must not lose our vigilance in interpreting the data—in all aspects of ophthalmological care, and certainly for biometric purposes.
Related: FDA approves faricimab to treat DME, wet AMD
References
1. Olsen T. Intraocular lens power calculation. J Cataract Refract Surg. 2009;35(12):2176-2177; author reply 2177-2178. doi:10.1016/j.jcrs.2009.07.021
2. Mylonas G, Sacu S, Buehl W, Ritter M, Georgopoulos M, Schmidt-Erfurth U. Performance of three biometry devices in patients with different grades of age-related cataract. Acta Ophthalmol. 2011;89(3):e237-e241. doi:10.1111/j.1755-3768.2010.02042.x
3. Pongsachareonnont P, Tangjanyatam S. Accuracy of axial length measurements obtained by optical biometry and acoustic biometry in rhegmatogenous retinal detachment: a prospective study. lin Ophthalmol. 2018;12:973-980. doi:10.2147/OPTH.S165875
4. Kanukollu VM, Agarwal P. Epiretinal membrane. In: StatPearls (Internet). StatPearls Publishing; January 2021. Updated July 30, 2021. Accessed January 9, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560703/
5. Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016;10:527-534. doi:10.2147/OPTH.S97722
6. Rajan MS, Bunce C, Tuft S. Interocular axial length difference and age-related cataract. J Cataract Refract Surg. 2008;34(1):76-79. doi:10.1016/j.jcrs.2007.08.023
7. Roy FH, Fraunfelder FW Jr, Fraunfelder FT. Roy and Fraunfelder’s Current Ocular Therapy. 6th ed. Saunders; 2008.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.