Why in-person care and technology must partner

A 74-year-old male was recently diagnosed with diabetes. He had been referred by his primary-care physician for ophthalmic evaluation.
Except for diabetes, the patient was not managed for other systemic diseases. His visual acuity was correctable to 20/20 in each eye. He was phakic and-with the exception of refractive correction-had no ocular history.
OCTA results
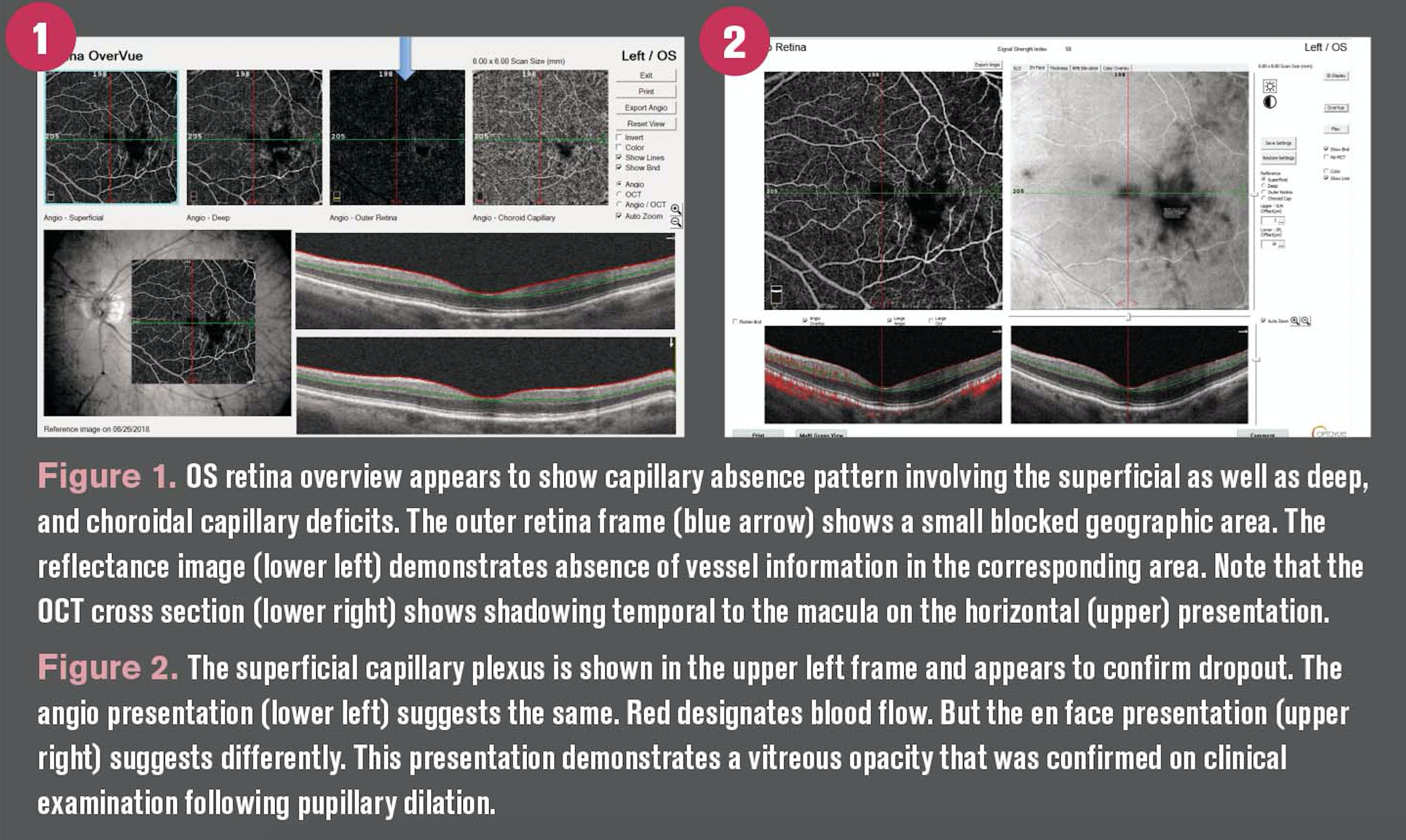
During pupillary dilation, the patient’s ocular coherence tomography angiography (OCTA) was obtained. The overview is shown in Figure 1. The angiograph is presented in Figure 2.
The first impression, given the diagnosis of systemic diabetes, was that these findings represented capillary deficiency.
Related: Using OCTA in optometric practice
Some of the data arguing against this interpretation is that the apparent capillary deficit continues through the entire retinal thickness. Projection artifacts are generally associated with anomalous vessel imaging.
Shortly after the introduction of commercial OCTA, projection artifacts confused interpretation. Software evolution has minimized those to a great extent.1,2
In this case, a vitreous opacity was responsible for obscuring visualization that at first view, given the patient’s systemic diagnosis, appeared to represent capillary absence. A vitreous floater was observed and confirmed on stereoscopic fundus examination following pupillary dilation.
Case points
This case illustrates a number of points.
First is the importance of live examination of the patient. I have heard it said that some specialists perform fundus evaluation as a “courtesy” to the patient.
Next is not jumping to a conclusion based on data from an evolving technology. Not all instrumentation is infallible.
In this case, the foveal avascular zone appears to be intact and of appropriate size. Newer analytical software (AngioAnalytics, which was FDA-cleared in June 2018) play a quantitative role in this.3
Since the imaging study was completed before the patient’s pupillary dilation had taken effect, there was suspicion that superficial capillary dropout would be present.
Related: The case of the scarred retina
This would potentially be consistent with the systemic diagnosis of diabetes. The initial observation heightened awareness for the possibility of more dire retinopathy being present.
With this in mind, the clinical evaluation initially focused specifically on that region of the macula.
However, before proceeding to the level of the retina, a large but asymptomatic vitreous floater was seen. By analyzing other frames of the angiograph, it became evident that the apparent capillary defect projected to other layers of the vasculature (see Figure 1).
Shadowing could also be seen on the cross-sectional presentation mirroring the floater (see Figure 2). What was initially a red flag became a red herring.
Future advancements
Automated image analysis for diabetic retinopathy has recently been FDA cleared.4 The clinical trials leading to the commercialization of this software have an interesting background. The interested reader is referred to several publications.5-7
In addition, automated analysis (artificial intelligence, deep machine learning) for age-related macular degeneration, glaucoma and cataract are on the horizon.8
Read more by Leo P. Semes, OD, FAAO
References:
1. Al-Sheikh M, Ghasemi Falavarjani K, Akil H, Sadda SR. Impact of image quality on OCT angiography based quantitative measurements. Int J Retina Vitreous. 2017 May 15;3:13.
2. Zhang Q, Zhang A, Lee CS, Lee AY, Rezaeki KA, Roisman L, Miller A, Zheng F, Gregori G, Durbin MK, An L, Stetson PF, Rosenfeld PJ, Wang RK. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmol Retina. 2017 Mar-Apr;1(2):124-136.
3. Optovue. Aids in early detection and management of diseases causing progressive blindness. Available at: https://www.optovue.com/news/optovue-receives-fda-clearance-for-angioanalytics-the-worlds-first-oct-angiography-metrics. Accessed 11/8/18.
4. Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. U.S. Department of Health and Human Services. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm604357.htm. Accessed 11/8/18.
5. Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a Clinically Useful Comprehensive Classification of Vascular and Neural Aspects of Diabetic Retinal Disease. Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):519-527.
6. van der Heijden AA, Abramoff MD, Verbraak F, van Hecke MV, Liem A, Nijpels G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018 Feb;96(1):63-68.
7. Li Z, Alzogool M, Xiao J, Zhang S, Zeng P, Lan Y. Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol. 2018 Oct;55(10):1075-1082.
8. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018 Sep 18;11(9):1555-1561.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.