Fitting and troubleshooting tips for scleral lens success
Increasing scleral lens use can benefit a larger patient base.

In 2021, eye care practitioners estimated scleral lenses accounted for approximately 14% of the gas-permeable (GP) lenses they prescribed, according to the Contact Lens Spectrum’s GP and Custom Soft Annual Report 2021.1 Although corneal lenses remain the dominant GP lens design, scleral lenses are becoming increasingly more popular among practitioners for a variety of corneal conditions.
Because scleral lenses are fitted to vault over both the cornea and limbus and land on the sclera, the resultant tear reservoir provides a plethora of advantages over traditional corneal lenses. These include superior vision, improved comfort, and greater ocular surface protection. Scleral lenses are usually considered as an option for patients with irregular corneas; however, they are also excellent options for patients with high ametropia and dry eye syndrome.

As scleral lenses have become commonplace in many practices, manufacturers have created simple fitting guides. When fitting empirically—regardless of the manufacturer—all scleral lenses follow the same 4 basic guidelines.
1. Choose a diagnostic lens
Choosing the correct diagnostic lens is crucial and includes 2 key steps: selecting proper design and selecting diameter. A prolate design is ideal for regular corneas or patients with corneal ectasias (eg, keratoconus and pellucid marginal degeneration). An oblate design is ideal for patients who have had refractive surgery (eg, LASIK, photorefractive keratectomy, or radial keratotomy). On occasion, topography reveals a corneal pattern that does not clearly fit into either category (eg, post transplant). In this case, try both designs and determine which is a better overall fit based on fitting guidelines 2 through 4.
Choosing the appropriate diameter should be guided by the patient’s horizontal visible iris diameter (HVID). This can be measured manually with an HVID ruler or slit lamp beam or obtained from metrics provided by a topographer. Once the appropriate diameter and design have been selected, choose a starting lens recommended by the manufacturer based on the patient’s corneal condition or keratometry values. When in doubt, choose a lens from the middle of the fitting set.
2. Assess apical and limbal clearance
Once the starting diagnostic lens is placed on the eye, evaluate the apical clearance. Most manufacturers recommend 100 to 250 μm of apical clearance after the lens has settled for approximately 30 minutes. Limbal clearance is equally as important as apical clearance. Ideally, aim for 50 to 100 μm.
Figure 1. Scleral lens demonstrating appropriate apical and limbal clearance.
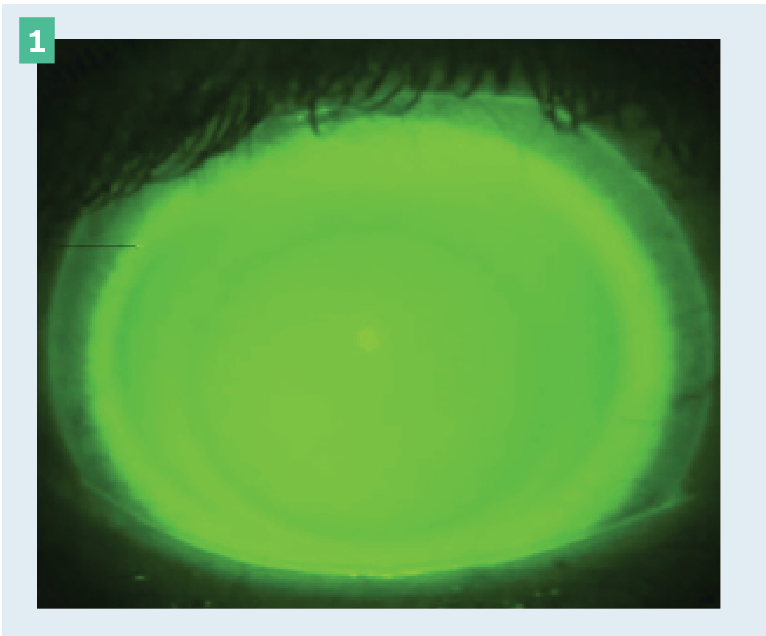
Both apical and limbal clearance can be evaluated with sodium fluorescein and blue light using the diffuse slit lamp beam and more precisely evaluated with white light and an optic section. In addition, the anterior segment feature on an ocular coherence tomographer (OCT) can aid in quantifying the exact amount of clearance both apically and limbally.
3. Evaluate the landing zone
After achieving the appropriate apical and limbal clearances, evaluate the scleral landing zone, which is the peripheral system of the scleral lens. The lens should align with the sclera and not exhibit any edge lift or vessel blanching.
4. Add the power
The final step is adding the over-refraction. It is wise to add the spherical over-refraction initially and then add the spherocylindrical over-refraction once the final fit of the lens has been established.
Figure 2. Variations of edge profiles.
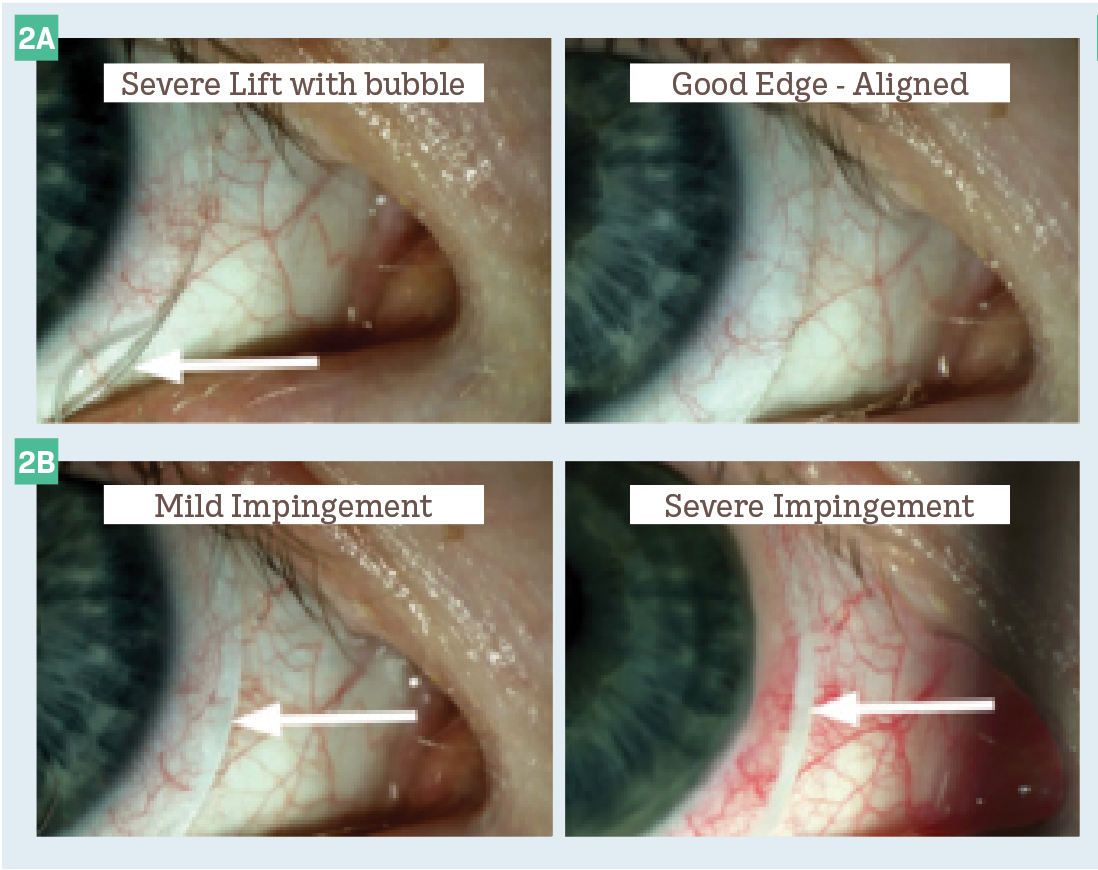
In a perfect world, following these 4 steps would provide each patient with an exact fit and exceptional vision (Figure 1). In reality, although the actual fitting of scleral lenses is simple, troubleshooting problems is more challenging. Here are some of the most common fitting challenges.
Conjunctival vessel blanching
This is caused by localized pressure on the conjunctiva and can be quadrant specific, meridional, or circumferential. Patients commonly report the following after removal: discomfort often extending into the next day, rebound hyperemia, and/or an impression ring. Blanching can be further broken down into 2 categories:
1. Impingement occurs along the outermost curve of the lens, causing the lens to dig into the conjunctiva. This cuts off circulation to the conjunctival vessels.
2. Compression occurs along the inner scleral landing curves and causes a whitening of the conjunctival vessels under the lens (Figure 2).
It is important to note that impingement and compression can occur concurrently. The most common causes of conjunctival vessel blanching are peripheral obstructions (eg, pterygium, pinguecula, or filtering blebs) and scleral asymmetry. To fix this issue, the simplest solution is to flatten the periphery of the lens in the area of blanching.
For peripheral obstructions, the fix may be a little more involved. Creating a notch around or a microvault over the obstruction will ensure a custom-tailored design that promotes overall ocular health and prevents unnecessary interaction with the obstruction.
Edge lift
This is caused by a lens fit flatter than the scleral profile and can also be quadrant specific, meridional, or circumferential. Patients will typically report lens awareness in the area of edge lift and potentially experience blurry vision due to bubbles and/or debris gathering under the lens. Edge lift can be viewed with a slit lamp and appear as a shadow under the area of lift (Figure 2).
Because the sclera is more spherical closer to the limbus and more toric toward the periphery, larger-diameter scleral lenses may be more challenging to fit than smaller-diameter onesThe Scleral Shape Study Group (SSSG) uncovered additional information regarding scleral shape. Approximately 65% of patients in the study achieved best fit with a back-surface quadrant-specific or free-form scleral lens design, 30% achieved best fit with toric landing zones, and only 6% achieved best fit using a scleral lens with a spherical landing zone.2
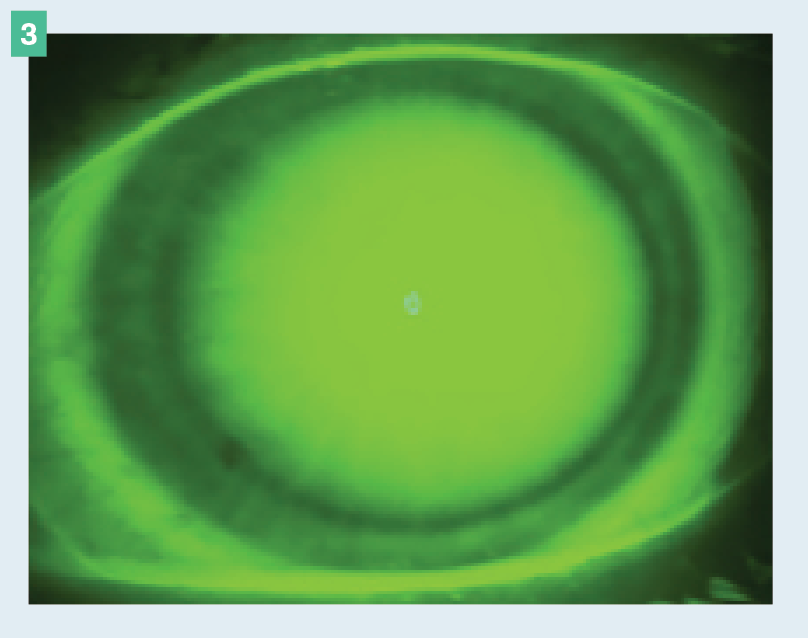
Figure 3. Limbal bearing seen in a 360° view.
Many manufacturers offer fitting sets that include diagnostic lenses with back-surface toric haptics and quadrant-specific haptics, making fitting these lenses much more approachable than in years past. Considering the findings from SSSG, most patients would benefit from these lens designs, and managing areas of blanching and/or edge lift would be minimized by using more customized haptics. For patients with especially irregular scleral profiles, free-form designs created from a scleral profilometer or impression-based design may be the better option.
Limbal bearing
This is a result of the limbal curve being fit too flat, typically causing a compression ring and/or limbal staining. Limbal bearing can result in corneal hypoxia and neovascularization. Because of this, any level of limbal bearing cannot be tolerated. Steepening the limbal curve, steepening the base curve, and increasing the overall diameter are all effective options for rectifying this issue. Limbal bearing is particularly problematic in patients with corneal grafts because it could potentially lead to rejection (Figure 3).
Conjunctival prolapse
Figure 4. Mild conjunctival prolapse.
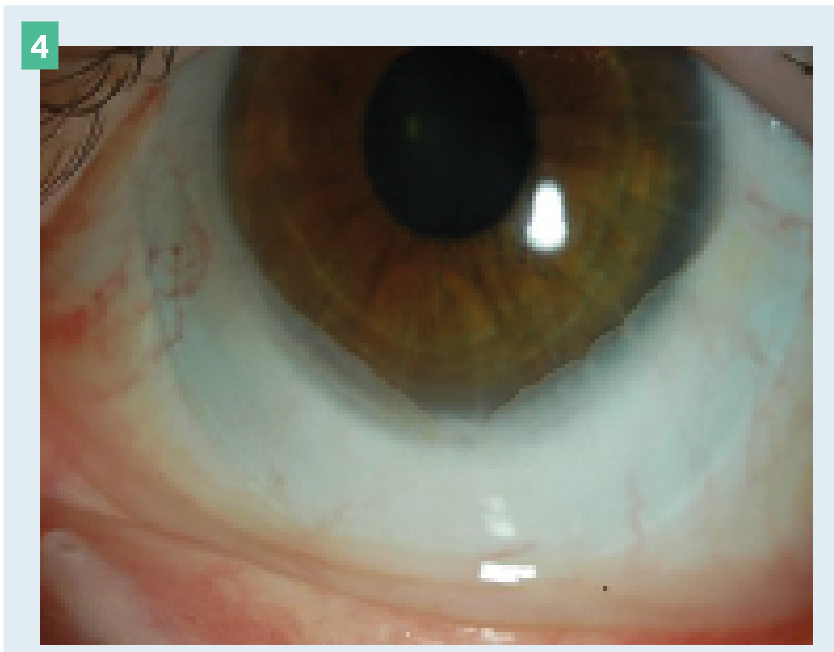
This is caused by entrapment of the conjunctiva near the limbal region between the scleral lens and the cornea. It is most commonly seen in older patients and is often completely benign. Monitoring the prolapse is typically all that’s necessary. However, if neovascularization or synechia develops, decreasing the lens diameter and/or decreasing the limbal clearance may improve the prolapse (Figure 4).
Debris/fogging
Studies have reported 26% to 46% of scleral lens wearers experience midday fogging.3 The exact etiology of this phenomenon is unknown, but it is likely associated with misalignment of the periphery, where the lens is either too loose or too tight.

Thankfully, now that toric haptics are becoming more conventional, practitioners are better equipped to tackle asymmetric scleral profiles. However, despite a perfectly aligned periphery, some scleral lens wearers still experience midday fogging. Other suggestions include addressing any ocular surface disease (OSD) before fitting, maintaining an appropriate amount of limbal clearance (50-100 μm), and using a mixture of preservative-free viscous solution and preservative-free saline.
Corneal staining
Because scleral lenses are designed to land on the sclera and not interact with the cornea, corneal staining is rare. A localized patch of staining typically indicates an area of bearing that would require an increase in clearance in that area. Diffuse corneal staining is usually a toxic reaction to entrapment of a preserved product under the lens. This can include filling solution, cleaning solution, or topical eye drops. Simply questioning the patient about their daily contact lens routine will likely reveal the culprit.
Finally, mechanical damage to the cornea during insertion and/or removal could also cause corneal staining. Extra instruction on proper application and removal can prevent future incidents.
Poor wetting
Figure 5. Scleral lens demonstrating poor wetting on the front surface.
(Images courtesy of Ashley Tucker, OD, FAAO, FSLS, Dipl ABO)
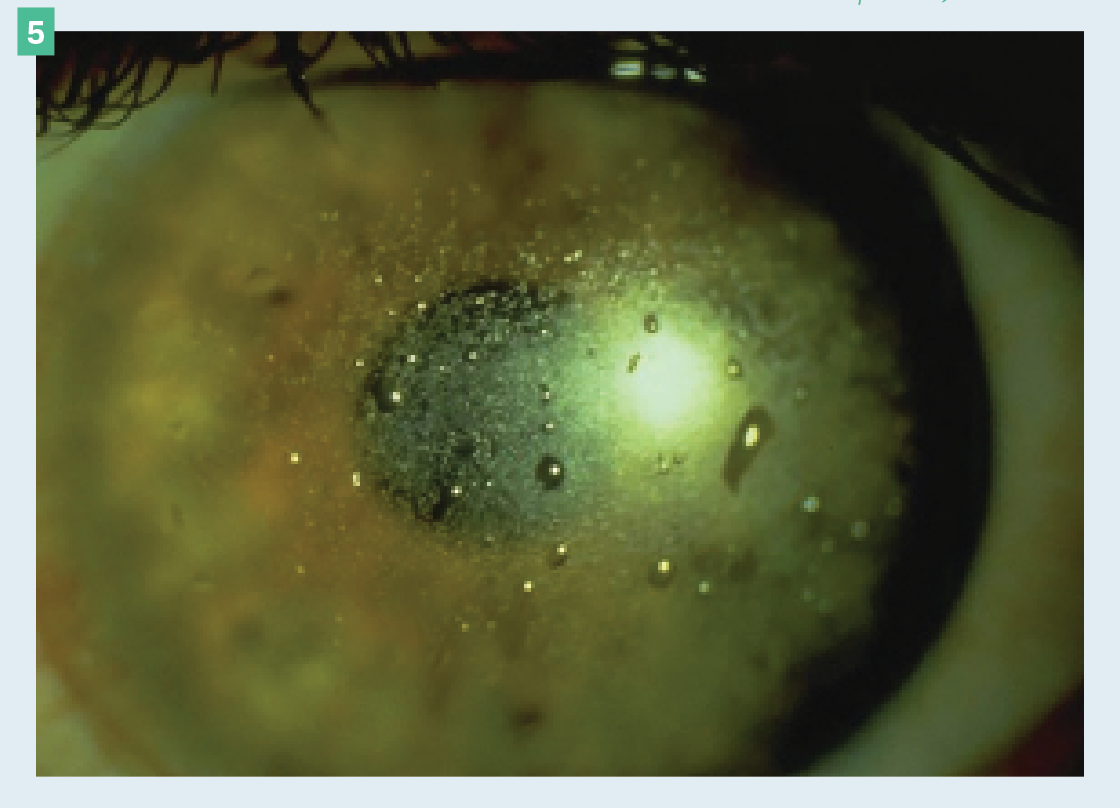
Mucoid, lipid, or protein deposits are normally the reason for poor wetting (Figure 5). These deposits can be caused by untreated OSD or external factors such as makeup, face creams, and hand soaps. Encourage patients to apply makeup and face creams after inserting the lens and only use mild, moisturizer-free soaps to wash their hands. Material choice also plays a role in proper wetting, so finding the appropriate balance between oxygen permeability and wetting angle is imperative.
Additionally, treating the lens with plasma and Tangible Hydra-PEG can enhance wettability and decrease deposits. Finally, practitioners should encourage patients to gently rub the front surface with nonabrasive cleaners to further reduce deposition.
References
1. Nichols JJ, Starcher L. Contact lenses 2021. Contact Lens Spectrum. January 1, 2022. Accessed September 17, 2022. https://www.clspectrum.com/issues/2022/january-2022/contact-lenses-2021
2. DeNaeyer G, Sanders DR, van der Worp E, Jedlicka J, Michaud L, Morrison S. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer: the SSSG study. J Contact Lens Res Sci. 2017;1(1):12-22. doi:10.22374/jclrs.v1i1.11
3. Fogt JS. Midday fogging of scleral contact lenses: current perspectives. Clin Optom (Auckl). 2021;13:209-219. doi:10.2147/OPTO.S284634

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.