Proper documentation helps assure prior authorizations
Newer dry eye medications require approval, and charting helps prove the need.
Prior authorizations, or PAs, are currently every medical optometrist’s nightmare, but there is hope if you know to plan ahead.
A PA according to the Center for Medicare and Medicaid Services (CMS) is “an approval...before you get care or fill a prescription.” The doctor must contact the patient’s insurance plan to show medical necessary reason for a particular drug or treatment for it to be covered.
Simply put, when ODs decide the medicine our patients need, the insurance is asking, “Do they really need it?” Addressing PAs can become a tiresome and frustrating process for the doctor, the staff, and the patient. However, with proper charting techniques and knowing the right language, ODs may be able to obtain approvals faster, with more consistentcy, and with fewer appeals.
Related: 6 steps to establish an OSD advocate in your practice
Medical need
A successful prior authorization process begins long before hitting the e-prescribe button. To prove a clear, medical need for a medication, detailed charting must back up the doctor’s recommendation. A PA can frequently be denied simply for a lack of supporting evidence. The purpose of the PA is to prove all other viable options have been exhausted, and there is a medical need for the medication.
With new medications, therapies, and treatments continually coming to market, it is more important than ever to document any and all prior treatments. From 2016 to 2019, 181 new medications were approved by the FDA,1,2 most notably for dry eye Xiidra (lifitegrast 5%, Novartis) and Cequa (cyclosporine 0.09%, Sun Pharma).
The recent increase in PA requests can be linked to increases in medication choices and more Rxs being written, leading to increased costs to medical insurances. Because of this, new medications are held to higher scrutiny during the PA process. By adding additional steps to approve Rxs and requiring generics or lower-cost mediations to be used first, insurers reduce upfront costs as well as make it harder to prescribe new medications.
Related: Dry eye in the digital age
Medications
A well-documented chart ready for PA approval shows that all medications, including over-the-counter (OTC) treatments, have been tried and listed clearly in the chart. Avoid non-specific documentation such as “continue present management.” Note under medications and in chart notes what medication the patient is using. The adage, “If you don’t note it in the chart, it didn’t happen,” applies perfectly here.
Dry eye PAs will often get denied for simple things like not trying over-the-counter tears at qid dosage. The doctor will counter saying the patient has been using them for years, but nothing is noted in the chart—which means there is no documentation to prove that statement.
Make sure doctors, technicians, or other staff write all medications by name and dose—and be specific. Do not list “artificial tears PRN,” or you are guaranteed a denial. Most insurances require documentation of 2 different OTC artificial tears used at least 2 weeks each with a dosage of 4 times a day. Without this documented in the chart, the PA will be denied, the patient will need to return to document failure of previous artificial tears, and the care plan goes backward.
It is important to list in the chart note adverse reactions to medications the patient may have. If the patient cannot handle the stinging from benzalkonium chloride (BAK) or has a preservative sensitivity, note it. This is especially helpful when the insurance compnay has a preferred formulary that does not work for the patient.
A separate point about medication charting is to describe why the doctor is switching medication. It can be as simple as “Formulary medication ineffective” or “Still dry with tears alone.”
When completing the PA, these words can help make the case for the patient.
Related: 10 new treatments in eye care
Testing
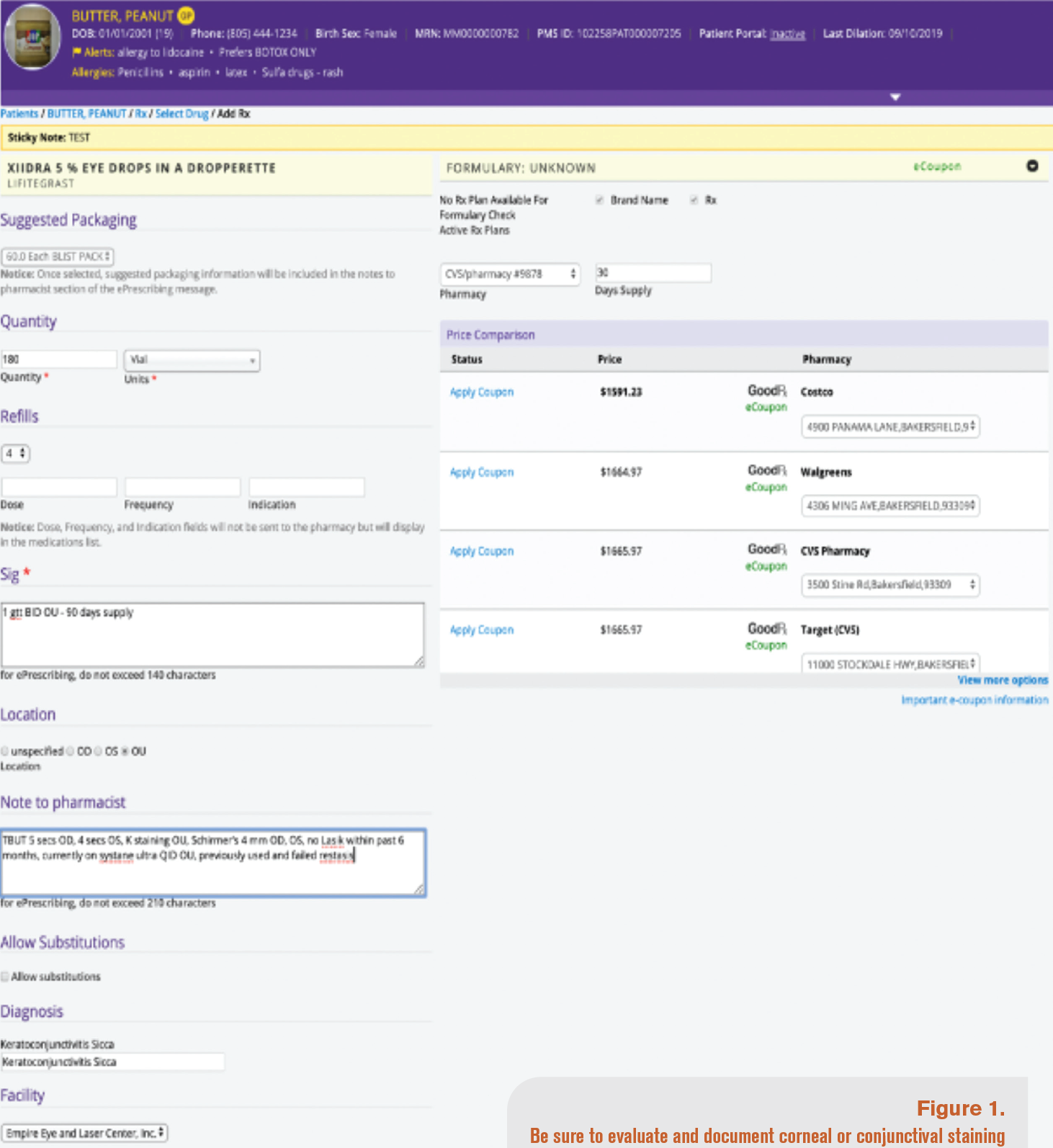
Testing is an integral part of charting for dry eye PA approval. In my experience, the more testing to document the condition, the better. At the very least, make sure to evaluate corneal or conjunctival staining with fluorescein or lissamine green and tear break-up time (see Figure 1). Chart notes must show how the patient has responded before and after treatment. This can demonstrate why the patient needs a change in therapy.
List treatments or procedures the patient has used. The more documentation of failed treatment, the more likely the patient will receive a quick approval. Something as simple as trying and failing warm compresses or punctal plugs can make a difference.
Related: Know the link between cotton wool spot and anemia
While waiting
Fortunately, most pharmaceutical companies will offer coupons and product samples to help patients through the PA process. Some companies, such as Novartis, have set up an extensive program (Xiidra iinsider within Ask iiris) to help with cost lowering and PA approval. This company, as well as many others, also offers a patient assistance program to deliver the medication to patients who cannot afford it.
Summing up
With thorough documentation and notes, the PA process can be faster and less frustrating. However, some insurance companies will fight PAs. Clearly document what the patient has tried and why the doctor thinks those treatments have failed. When the insurance company is reviewing charts for keywords to base its approval or denial, it is important to use the terminology it is looking for. Such terminology varies based on the medication, but the insurance company is looking for proven therapeutic failure or intolerance of accepted formulary options.
In the case of exceptionally difficult PAs, the doctor may appeal the decision, and a well-worded letter of appeal along with exemplary charting will get the job done.
More by Dr. Dang: How to incorporate nutrition into dry eye practice
References:
1. U.S. Food & Drug Administration. Novel Drug Approvals for 2019. Available at: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019. Accessed 5/27/20.
2. Dalton M. Understanding prevalence, demographics of dry eye disease. Ophthalmology Times. Available at: https:// www.ophthalmologytimes.com/article/ understanding-prevalence-demographics-dry-eye-disease. Accessed 5/27/20.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.