The vitreous—remember me?
Watching out for inevitable degenerative changes can improve patient care.
Image credit: AdobeStock/Parilov
At the Great Western Council of Optometry meeting in 2022, Leo Semes, OD, FAAO—an optometric retina pioneer and frequent contributor to this journal—gave a lecture called “I Care About the Vitreous and You Should, Too.” His talk made sense; there is no disputing that everyone has a vitreous, which is guaranteed to go through degenerative changes and possibly lead to patient complaints of flashes or floaters, or visual sequelae from vitreomacular interface abnormalities through the evolution of a posterior vitreous detachment (PVD). This brings a lot of people into our offices and emphasizes Semes’ lecture title; we should pay attention to the vitreous.
Though not completely understood, the vitreous has several proposed functions, such as acting as a shock absorber for surrounding tissues, providing transparency, and contributing to the growth of ocular tissues during development.1,2 It may also play a role in regulating oxygen levels where the viscoelastic properties could limit the higher concentration of oxygen needed for the metabolically active retinal pigment epithelial (RPE) cells from reaching the oxygen-sensitive lens epithelial cells.1 The vitreous contains elevated levels of the antioxidant vitamin C, but there is a lack of clear evidence that it protects against reactive oxygen species. Also absent are studies that support the hypothesis that dietary vitamin C supplementation translates to a higher vitreous concentration of the antioxidant.3
Anatomically, the vitreous is 4 ml in volume and surrounded by a cortex of densely packed collagen, which is thinnest at the macula. To put the volume in perspective, 4 ml equates to 10 preservative-free artificial tear vials. The vitreous contains 98% water with the rest being structural proteins, mainly collagen in the form of fibrils, and hyaluronan, a glycosaminoglycan.3 The fibrils bundle together to form an interconnected network that plays a key role in keeping the vitreous in a gel state.2 Hyaluronan entangles itself within this network and attracts water, which provides a swelling pressure to the fibrils and contributes to the vitreous’ viscoelasticity.1 The fibrils orient themselves in different directions depending on their location. They run in an anterior-posterior direction and insert perpendicularly at the vitreous base where their density is highest. This explains the relatively unbreakable adhesion in that area.2
In other regions of the eye, however, the collagen fibers run parallel to the vitreoretinal surface and don’t directly insert into the retinal surface. Therefore, the adhesive force between the posterior vitreous cortex and the internal limiting membrane (ILM) in these areas may be due to a glue-like layering of laminin, fibronectin, chondroitin, and heparan sulfate proteoglycans.4 This adhesion is strongest at sites where either the posterior vitreous cortex or the ILM is thinnest. For the ILM, this includes the blood vessels, optic nerve margins, and an area 500 µm to 1500 µm around the fovea.4 The posterior vitreous cortex is also thinnest in all those places except the optic nerve, where it is absent, which is why the Weiss ring has its characteristic appearance.
As mentioned before, the vitreous degenerates with age and undergoes both synchysis and syneresis. Synchysis is vitreous liquefaction and the formation of liquified pockets, which often occur first in the central vitreous and in front of the macula.5 This may be due to the dissociation of hyaluronan from collagen.4 Most patients aged over 70 years have at least 50% vitreous gel liquefaction, which can accelerate in high myopia, trauma, inflammation, or intraocular surgery.5,6 Syneresis, on the other hand, is a collapse of the collagen network and an aggregation of collagen fibrils (also known as myodesopsia or floaters).7
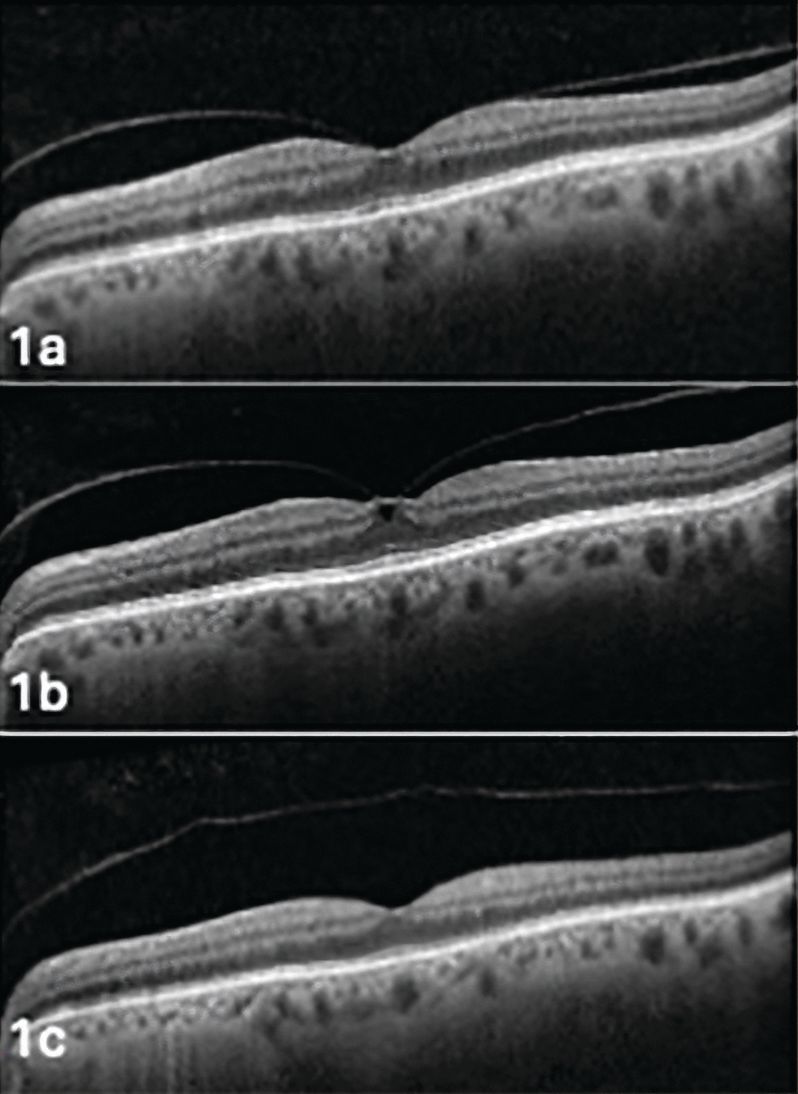
Figure 1. Evolution in the same patient of vitreomacular adhesion (Figure 1a) to vitreomacular traction (Figure 1b) to posterior vitreous detachment (Figure 1c). Image courtesy of Jim Williamson, OD, FAAO, FORS.
The endgame of these processes is the evolution of the PVD, which generally occurs between the ages of 40 and 60 years. With liquefaction, the vitreous gel collapses and contracts. When this happens in concert with dehiscence of the vitreoretinal adhesion, an innocuous PVD forms.8,9 Alternately, an anomalous PVD may occur when the vitreoretinal adhesion remains intact while the vitreous pulls away.5 This produces tugging, which may cause a plethora of complications such as vitreomacular and vitreopapillary traction, macular hole, retinal tear, or hemorrhage, among others.
Figure 2. A slit lamp biomicroscopy view of a complete posterior vitreous detachment with collapse (Figure 2a). Make sure to have full illumination and focus posteriorly. Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 2. Adding a fundus lens allows an even more posterior view, which here shows the Weiss ring (Figure 2b). Remember that there is no posterior vitreous cortex over the optic nerve, which gives the Weiss ring its characteristic shape. Image courtesy of Jim Williamson, OD, FAAO, FORS.
Because there are sites of stronger vitreoretinal adhesion, the PVD process occurs in stages (Figure 1). The vitreous first detaches in the perifoveal area, followed by release of the vitreofoveal adhesion in stage 2. Stage 3 denotes a near-complete PVD with only vitreopapillary adhesion. Detachment of this final area marks a stage 4 or complete PVD.5 Clinically, this will appear as a Weiss ring over the optic nerve or the ability to view the posterior vitreous cortex using the slit lamp (Figure 2).10 The Weiss ring, however, does not confirm that there may still be attachments in the peripheral retina.11
Figure 3. Vitreoschisis may occur anteriorly to the hyalocytes, which results in a more equal-appearing thickness of the separation (Figures 3a and 3c). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 3. When the vitreoschisis occurs posterior to the hyalocytes, a thinner membrane remains (Figure 3b). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 3. Vitreoschisis may occur anteriorly to the hyalocytes, which results in a more equal-appearing thickness of the separation (Figures 3a and 3c). A break in the posterior vitreous cortex appears in Figure 3c (arrow). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Sometimes during an anomalous PVD, a vitreoschisis may occur where the posterior vitreous cortex splits, which leaves the cortex’s outermost layer still attached to the retina. On optical coherence tomography (OCT), this appears as 2 layers joining into 1, forming a lambda (l) or Y sign (Figure 3).12 The posterior vitreous cortex contains hyalocytes, and when the split occurs posterior to them, a thin membrane remains. If the break occurs anteriorly, the hyalocytes stay put. Researchers suggest a role with the former in macular holes and the latter with macular pucker and tangential vitreoretinal contraction.12
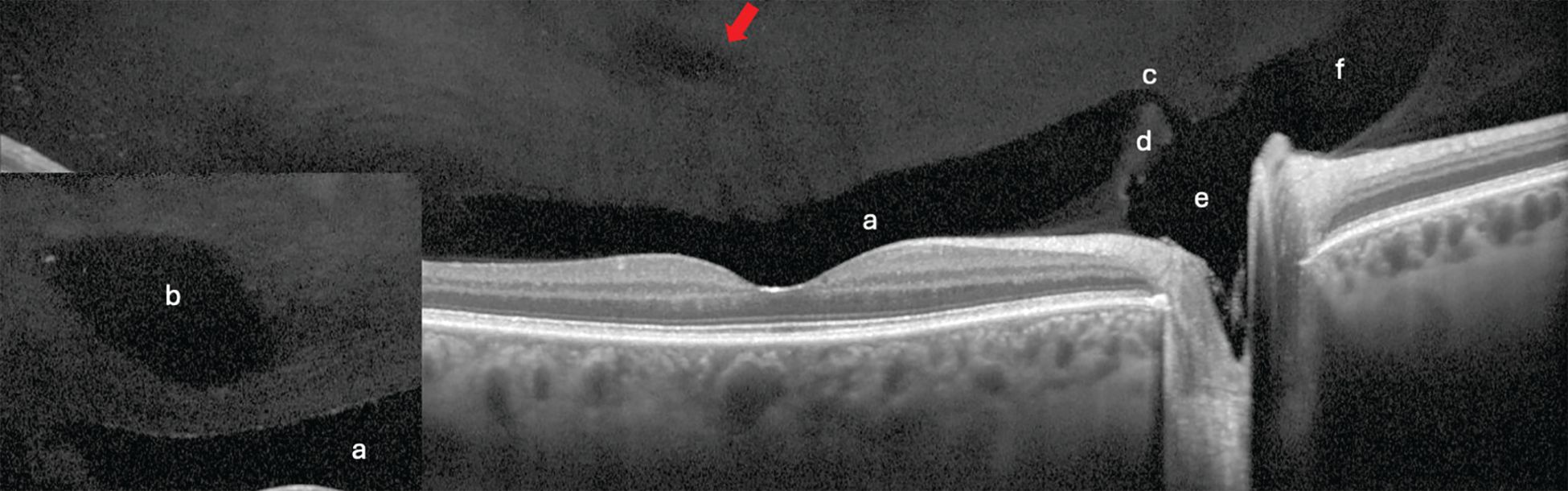
Figure 4. Anteriorly focused widefield Spectralis optical coherence tomography of 2 patients. a) Posterior precortical vitreous pocket (PPVP). b) A prominent supramacular bursa (inset) versus a more subtle one (arrow). c) Only about half of eyes have a collecting channel. d) Septum e) Martegini area f) Cloquet’s canal. Image courtesy of Jim Williamson, OD, FAAO, FORS.
Historically, the clinical evaluation of the transparent vitreous proved challenging to early optometrists armed only with a direct ophthalmoscope or B-scan ultrasound. Now, practitioners have the slit lamp to focus on the anterior vitreous, and by using a fundus lens, the posterior vitreous as well. Binocular indirect ophthalmoscopy (BIO) expands the view through the vitreous periphery.
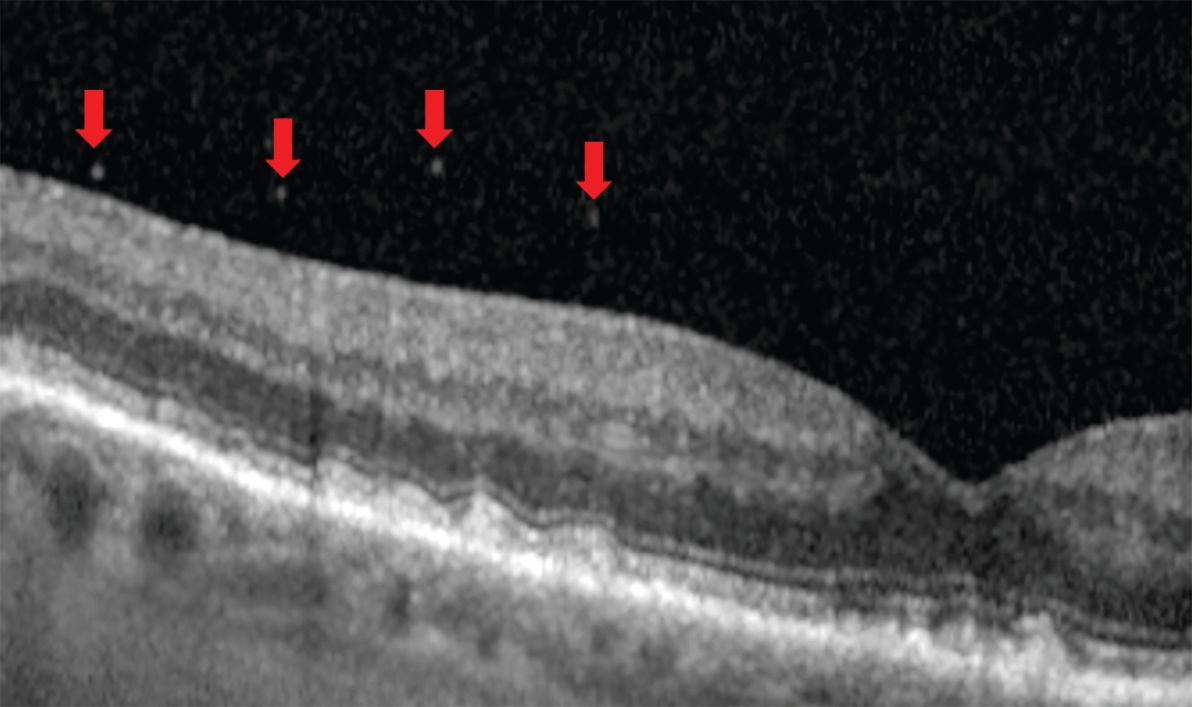
Figure 5. Ultrawidefield optical coherence tomography (OCT) through an area of dark without pressure (Figure 5a) shows an attenuated ellipsoid zone (Figure 5b, circle). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 5. Ultrawidefield optical coherence tomography (OCT) through an area of dark without pressure (Figure 5a) shows an attenuated ellipsoid zone (Figure 5b, circle). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 5. The opposite occurs in white without pressure (Figure 5c, circle) where the ellipsoid zone is highly reflective. Thus, OCT demonstrates that white without pressure is not a vitreoretinal interface abnormality. Image courtesy of Jim Williamson, OD, FAAO, FORS.
Detailing the structure, however, required other methods. Early work included scanning electron microscopy and then fluorescein staining of postmortem eyes.9 Time-domain OCT (TD-OCT) added an in vivo vitreous view unlike anything previously seen. Today, higher-resolution spectral-domain OCT (SD-OCT) and swept-source OCT (SS-OCT) allow the detection of more specific features (Figure 4). Further, the employment of OCT in the retinal periphery debunked the previously held belief of a “vitreoretinal interface change” as the cause for dark or white without pressure and proved the source to be an attenuated or hyperreflective ellipsoid zone (Figure 5).13
One of the most interesting vitreous findings of early investigators is the posterior precortical vitreous pocket (PPVP), also sometimes referred to as the premacular bursa or just bursa, which means sac or pouch (Figure 4). Located above the macular area, this dome- or boat-shaped liquified space measures on average about 0.7 mm high and 6.4 mm wide.14 Its posterior border contains a thin layer of vitreous cortex while the vitreous gel outlines the anterior border.9 Therefore, the PPVP spares the premacular vitreous cortex from anterior gel contraction, which plays a key role during vitreous degeneration.14 It also explains the “wolf’s jaw” appearance of fibrovascular proliferation in proliferative diabetic retinopathy. The superior and inferior temporal locations along the arcades coincide with the wall of the PPVP (Figure 6). If bleeding into the PPVP occurs, the hemorrhage will take on a boat-shaped appearance as it collects at the wall’s bottom due to gravity.
Figure 6. The “wolf’s jaw” appearance of fibrovascular neovascularization due to it bordering the wall of the precortical vitreous pocket (Figure 6a). Image courtesy of Jim Williamson, OD, FAAO, FORS.
Figure 6. Bleeding into the precortical vitreous pocket produces an arcuate hemorrhage (Figure 6b). Image courtesy of Jim Williamson, OD, FAAO, FORS.
With the anatomy behind us, there are a couple of clinical points to remember. When examining those with acute symptoms, don’t always expect a complete PVD. Over 25% may present with the incomplete variety.11 In either case, it is vital to document the presence or absence of vitreous hemorrhage (VH) and lattice degeneration. The likelihood of a retinal tear appears to coincide with the amount of VH.15 For lattice, the vitreous is firmly attached at its edges, thus increasing both the tractional force and the risk for a retinal break.
Performing a dilated peripheral retinal examination using a BIO with scleral depression is still the gold standard for assessment as the rates of initial retinal breaks across studies hover around 10%.16 A recent study concluded that ultrawidefield imaging alone failed to detect nearly 50% of horseshoe tears.17
Figure 7. The stardust sign as seen in a patient found to have a vitreous hemorrhage. Image courtesy of Jim Williamson, OD, FAAO, FORS.
Finally, OCT remains paramount in the assessment of patients who present with vitreous and vitreomacular interface abnormalities. A widefield scan tends to give a better overall view since it includes both the macula and optic nerve. Scanning protocols such as the 5-line raster, or fast scan, may fail to detect an anomaly due to their limited slices through the fovea. The International Vitreomacular Traction Study Group states that only one OCT-B scan is required to define vitreomacular adhesion, vitreomacular traction, or full-thickness macular hole.18 Therefore, a 24- or 48-line radial scan centered on this area should be performed to capture the entirety of the macula. Also, don’t forget to look up into the vitreous when analyzing the OCT-B scans to search for focal, round reflections, which may indicate red blood cells indicative of a VH. Several authors termed this the “stardust sign” and found retinal tears in over 25% of those patients (Figure 7).19
Being familiar with these vitreous details certainly helps us look after our patients. Thanks to Semes, we now remember the importance of the vitreous.
References:
Tram NK, Maxwell CJ, Swindle-Reilly KE. Macro- and microscale properties of the vitreous humor to inform substitute design and intravitreal biotransport. Curr Eye Res. 2020;46(4):429-444. doi:10.1080/02713683.2020.1826977
Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye (Lond). 2008;22(10):1214-1222. doi:10.1038/eye.2008.21
Sebag J. Vitreous and vitreoretinal interface. In: Sadda SR, Schachat AP, Wilkinson CP, et al. Ryan’s Retina, Volume 1. Elsevier; 2022:534-571.
Milston R, Madigan MC, Sebag J. Vitreous floaters: etiology, diagnostics, and management. Surv Ophthalmol. 2016;61(2):211-227. doi:10.1016/j.survophthal.2015.11.008
Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371-382.e1. doi:10.1016/j.ajo.2009.11.022
Schwartz DM, Shuster S, Jumper MD, Chang A, Stern R. Human vitreous hyaluronidase: isolation and characterization. Curr Eye Res. 1996;15(12):1156-1162. doi:10.3109/02713689608995150
Los LI, van der Worp RJ, van Luyn MJA, Hooymans JM. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44(7):2828-2833. doi:10.1167/ iovs.02-0588
Sebag J. Posterior vitreous detachment. Ophthalmology. 2018;125(9):1384-1385. doi:10.1016/j.ophtha.2018.05.018
Kishi S. Vitreous anatomy and the vitreomacular correlation. Jpn J Ophthalmol. 2016;60(4):239-273. doi:10.1007/s10384-016-0447-z
Kakehashi A, Takezawa M, Akiba J. Classification of posterior vitreous detachment. Clin Ophthalmol. 2014;8:1-10. doi.org/10.2147/OPTH.S54021
Carrero JL. Incomplete posterior vitreous detachment: prevalence and clinical relevance. Am J Ophthalmol. 2012;153(3):497-503. doi:10.1016/j.ajo.2011.08.036
Gupta P, Yee KMP, Garcia P, et al. Vitreoschisis in macular disease. Br J Ophthalmol. 2011;95(3):376-380. doi:10.1136/bjo.2009.175109
Yu H, Luo H, Zhang X, Sun J, Zhong Z, Sun X. Analysis of white and dark without pressure in a young myopic group based on ultra-wide swept-source optical coherence tomography angiography. J Clin Med. 2022;11(16):4830. doi.org/10.3390/jcm11164830
Kishi S. Impact of swept source optical coherence tomography on ophthalmology. Taiwan J Ophthalmol. 2016;6(2):58-68. doi.org/10.1016/j.tjo.2015.09.002
Flaxel CJ, Adelman RA, Bailey ST, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration preferred practice pattern. Ophthalmology. 2020;127(1):146-181. doi.org/10.1016/j.ophtha.2019.09.027
Uhr JH, Obeid A, Wibbelsman TD, et al. Delayed retinal breaks and detachments after acute posterior vitreous detachment. Ophthalmology. 2020;127(4):516-522. doi.org/10.1016/j.ophtha.2019.10.020
Lin AC, Kalaw FGP, Schönbach EM, et al. The sensitivity of ultra-widefield photography versus scleral depressed examination for detection of retinal horseshoe tears. Am J Ophthalmol. 2023;255:155-160. doi:10.1016/j.ajo.2023.07.010
Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611-2619. doi:10.1016/j.ophtha.2013.07.042.
Itakura H, Itakura M, Sato T. Stardust sign and retinal tear detection on swept source optical coherence tomography. Retina. 2022;42(2):336-339. doi:10.1097/IAE.0000000000003317

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.