Travoprost eluting drug-delivery device shows promise
In the chronic treatment of intraocular pressure (IOP) lowering in glaucoma patients, eye care has experienced a renaissance in pharmacologic molecules as well as delivery modalities.
For topical treatment in glaucoma, novel molecules, preservative-free drop options, and compounded medications have increased the glaucoma drop options for patients.
Unfortunately, whether due to cost, side effects, or a number of other causes, patient non-compliance is the practitioner’s biggest concern with glaucoma drops.
According to a retrospective study, in more than 5,000 patients who were prescribed glaucoma drops, nearly 50 percent stopped taking their drops by six months, and just over one third of patients had a recent refill on file at three years after starting the medication.1
In another study, in over 1000 patients newly prescribed topical glaucoma drops, only 20 percent of patients had persistently good drop adherence at the one-year time point, and this number dropped to 15 percent at four years.2
Previously by Dr. Ibach: Best practices for comanaging crosslinking patients
Glaucoma drug delivery
Drug delivery devices throughout eye care, but specifically for glaucoma, is an expanding arena showing real potential.
Glaucoma drug delivery is fast evolving; there are non-surgical external modalities, such as Allergan’s bimatoprost ring; an intracanalicular plug from Ocular Therapeutix; as well as surgically implanted devices such as Envisia Therapeutics’ ENV515, Allergan’s Bimatoprost SR, and Glaukos’ iDose.3
It is important to note that the above-mentioned technologies are not U.S. Food & Drug Administration (FDA) approved at this time but in clinical trials.
Related: Glaucoma: Which drops should you use?
I was a sub-investigator in both Phase II and Phase III of the FDA clinical trial for iDose, a travoprost-eluting implantable device. Interim results of the Phase II trial are available.
The purpose of the trial was to evaluate the safety and efficacy of two novel travoprost sustained-release drug delivery devices (iDose fast elution and iDose slow elution) in comparison to timolol for patients with open-angle glaucoma (OAG) and ocular hypertension (OHTN). The primary hypothesis was both iDose fast elution rate and iDose slow elution rate will be as effective as timolol in lowering IOP. The secondary hypothesis was both implants will have a favorable safety profile and be well tolerated.3
Related: Involve patients in monitoring IOP
Study design
This study is a three-year randomized prospective Phase-II double masked clinical trial, which is still conducting follow-up visits. Some 154 patients were randomized 1:1:1 into iDose fast, iDose slow, or placebo group of timolol twice per day (bid).4
Note that both iDose groups instilled a “sham” drop of artificial tears bid to maintain study quality.4
The primary endpoint, which has been met in all patients, is IOP lowering at 12 weeks post-implantation. The secondary endpoint is IOP lowering at one-year post surgery.4
The inclusion criteria for this trial included patients who are ï³18 years old with phakic or pseudophakic lens status. Patients included had mild-moderate OAG or OHTN (including pigmentary dispersion and pseudo-exfoliative glaucoma) on 0 to 3 medications at baseline visit. Inclusion criteria required a washed-out IOP 21 to 36 mm Hg.
Pertinent exclusion criteria were traumatic, uveitic, neovascular, or angle-closure glaucoma. Corneal, retinal, or systemic conditions that could confound results were also excluded.4
Related: 9 things glaucoma patients want to know
Implant and surgery
iDose is an implantable sustained-release drug delivery device that releases a proprietary formulation of travoprost. The titanium implant measures 1.8 mm by 0.5 mm and is implanted into the anatomical drain of the eye at the level of the trabecular meshwork (TM).
The implant has three main parts: the scleral anchor which anchors into the TM, the drug reservoir that serves at the body of the device, and the elution membrane which titrates the travoprost release. The travoprost therapeutic index is achieved with micro amounts of the drug because corneal permeability is bypassed.4
The surgical procedure was tightly controlled and monitored. iDose implantation is a stand-alone procedure performed in one eye. All patients were given anesthesia and brought into the operating room (OR). Randomization took place in the OR, and all patients were equally prepared for surgery. Using a gonioprism with an ab-interno approach, the surgeon implanted the device with the scleral anchor fastening into the TM. Postoperative visits were conducted at postop one day, postop one week, postop one month, and postop three months in the primary subset and endpoint.5
Related: Diagnosing and managing patients with narrow angles
ResultsDemographics and preop characteristics
Across all three study groups, patient demographics were balanced. Averaging all three groups, the mean age at the time of enrollment was 62.7 years. The most common patient race was Caucasian.
Disease type overwhelmingly favored OAG over OHTN at a rate of over 3:1. In all three groups, the most common number of glaucoma medications was one. In all three groups, visual field loss at time of enrollment was low with a mean deviation (MD) below three in all groups.
See Table 1 for complete study patient demographics.4
IOP and medication lowering
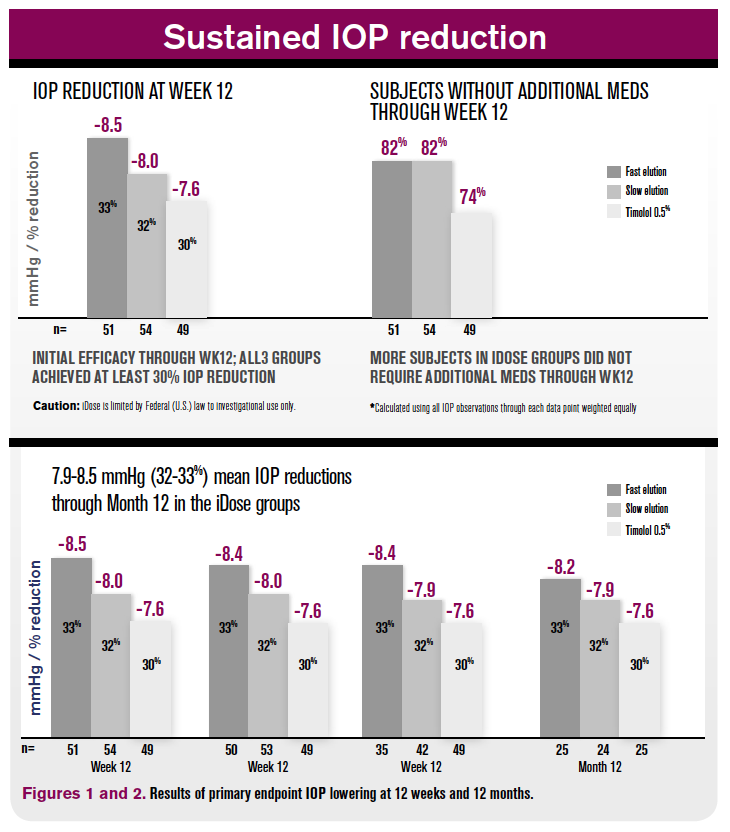
At the 12-week primary endpoint, mean IOP lowering was 8.5 mm Hg (33 percent reduction), 8.0 mm Hg (32 percent), and 7.6 mm Hg (30 percent) in groups iDose fast-elution, iDose slow-elution, and timolol 0.5%, respectively (Figure 1).
Subjects without additional medications through the primary endpoint was 82 percent in both iDose fast and slow, compared to 74 percent in the control group.
At the secondary endpoint of 12 months post-implantation, both iDose fast and iDose slow maintained IOP lowering of 32 to 33 percent from baseline (Figure 2). Out to one year post-implantation, both iDose fast elution and slow elution were able to keep mean medications at or below 0.5.4
Safety outcomes
For patients in both iDose fast and iDose slow groups, the safety profile was favorable with no intraoperative or serious adverse events.
Conjunctival hyperemia was not reported in either implant arm. In this study, patient blood was drawn to look for systemic serum levels of travoprost, and in both implant arms travoprost was not detected in patient blood serum.3
Complete ocular adverse events at 12 weeks post-op shown in Table 2.
Discussion
Medical treatment in glaucoma remains a mainstay for this chronic disease. Innovation in drug molecules is important because topical drops still have a high adoption rate by patients.
In a patient questionnaire of 126 patients, 55 percent of stable patients desired to continue topical drops over alternative therapies, including laser therapy, external or internal drug delivery, or intraocular surgery. Specifically comparing topical drops vs. a drug-delivery device that provided therapy for three to six months, it was nearly an equal 50-50 split on treatment preference.6
Drug-delivery devices used in glaucoma treatment remove the element of non-compliance as long as the devices are correctly inserted or implanted. For many practitioners, a utopic view of continuous medical treatment that may avoid IOP spikes due to missed or poorly timed drop administration is desirable.
Related: Quality-of-life metrics improve glaucoma staging
Two differentiating factors of this specific travoprost-eluting implant are safety and duration of efficacy. Paramount in any glaucoma treatment but magnified in mild OAG and OHTN patients who have minimal to no glaucoma symptoms is providing a safe treatment with minimal adverse events.
As shown above, the safety profile of iDose implantation is favorable. Conjunctival hyperemia scores of zero in both implant groups favors intraocular travoprost compared to topical prostaglandins for hyperemia scores. In both iDose fast and iDose slow, the IOP-lowering help at 32 to 33 percent out to one year post-implantation.
iDose sustained-release implants have shown promise in the treatment of OHTN and mild-moderate OAG. Phase III clinical trials are underway in the U.S., and I look forward to having this treatment option for glaucoma patients in the future.
Read more about glaucoma
References:
1. Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005 Oct;140(4):598-606.
2. Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015 Oct;122(10):2010-21.
3. Schweitzer J, Ibach M. Sustained-release drug delivery: The future of glaucoma treatment. Glaucoma Today. Available at: http://glaucomatoday.com/2016/12/sustained-release-drug-delivery-the-future-of-glaucoma-treatment/. Accessed 4/30/19.
4. Ibach M. Interim results of a prospective Phase II study of Travoprost Intraocular Implants. Paper presented at American Academy of Optometry annual meeting; Nov. 7-11, 2018; San Antonio, Texas.
5. Glaukos Corporation. Prospective, randomized Phase II study comparing two elution rates of Glaukos Travoprost Intraocular Implants to Timolol Maleate ophthalmic solution, USP, 0.5%. Available at: https://clinicaltrials.gov/ct2/show/NCT02754596. Accessed 4/30/19.
6. SooHoo JR, Golas, L, Marando C, Seibold LK, Pantcheva M, Ramulu PY, Kahook MY. Ophthalmology. 2016 Jul;123(7):1621-2.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.