Vision rehabilitation of patients with traumatic brain injury
A protocol of patient questions and clinical measurements can show a need for therapy

According to the CDC, traumatic brain injury (TBI) is a leading cause of death and disability in the US. Each year an estimated 1.5 million Americans sustain a TBI. From 2006 to 2014, the number of TBI-related emergency department visits, hospitalizations, and deaths increased by 53%.1 According to the US Department of Defense, up to 75% of the armed forces that suffered a TBI reported visual changes.2
Based on these data, approximately 1.1 million Americans with TBI may visit eye care practitioners’ offices each year reporting significant changes to quality of life and requiring assistance with activities of daily living.
Several types of visual dysfunctions are common after effects of TBI. Blast injury, penetrating injuries, and blunt trauma may cause structural damage to the eye or lesions or swelling in the brain that can disrupt visual pathways.3 In communicating with patients and patient care teams, it is important to help non-eye care providers understand that the majority of the sequelae to TBI is not ocular related but related to changes in the brain and the pathways that control the eyes.
Between 2000 and early 2016, over 347,962 TBIs were diagnosed in the U.S. armed forces, with approximately 80% classified as mild TBIs (mTBI).3
In a study to determine the prevalence of vision dysfunctions after mTBI, 100 patients were examined, and 69% had 1 or more of the following vision diagnoses:
– Accommodative disorders (51%)
– Convergence insufficiency (49%)
– Saccadic dysfunction (29%)
In all, 46% of patients had more than 1 vision diagnosis.4 For review of visual changes in moderate to severe brain injury including visual field loss, please refer to part 1 of this series (“Vision rehabilitation of patients with stroke,” November 2020).
Most mTBI symptoms resolve after 28 days.5 If a patient presents with lingering concerns, The national Vision Center of Excellence provides an evidence-based algorithm of care for ophthalmology and optometry in patients reporting visual changes post-mTBI.3 Often, these patients require 2 visits for the clinician to gather all pertinent data to provide optometric vision therapy. For the primary care clinician, the following protocol is useful.
Exam protocol
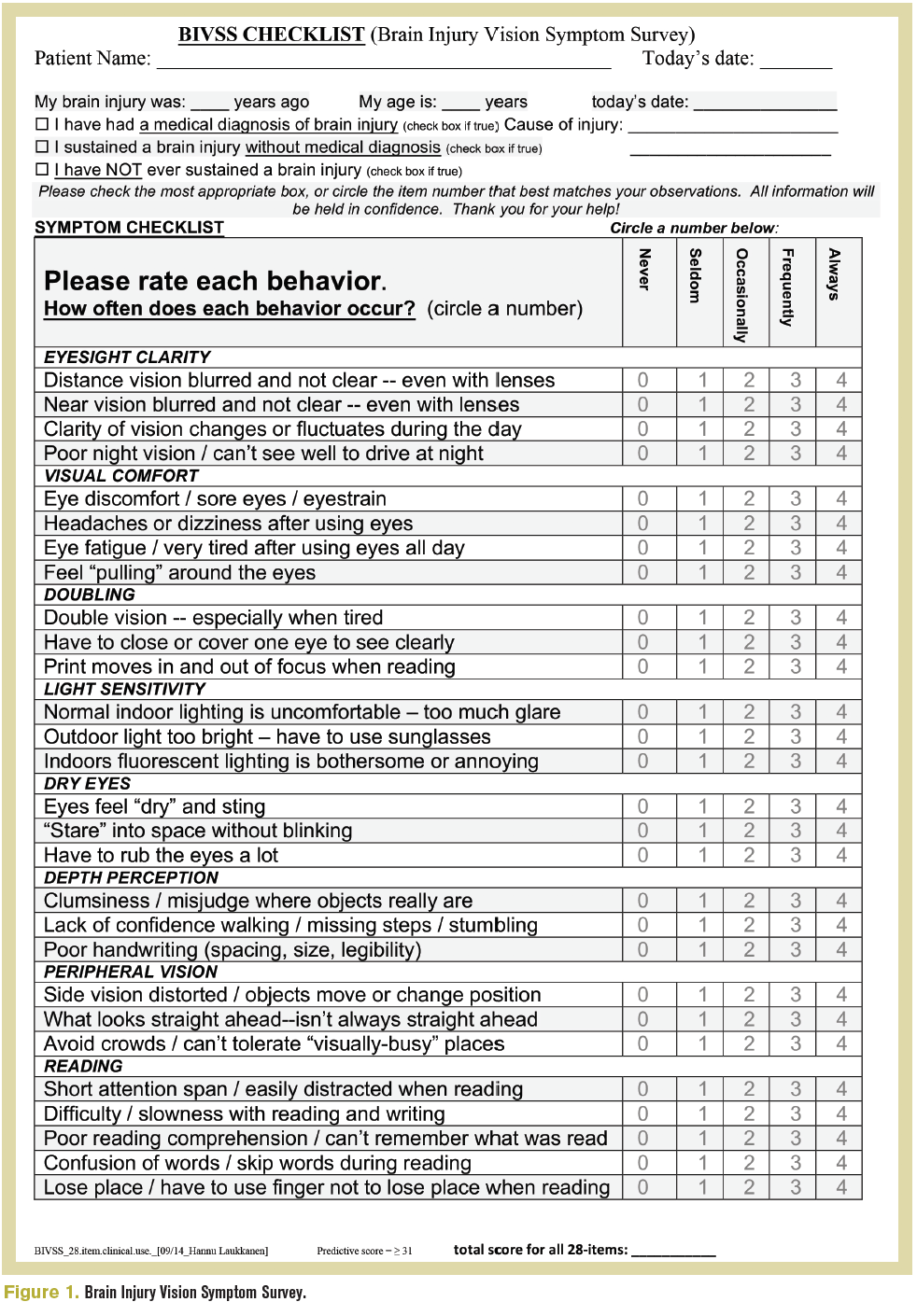
A comprehensive eye exam is the foundation for a detailed functional vision assessment, all beginning with a detailed case history. The Brain Injury Vision Symptom Survey (Figure 1) can guide the new or unfamiliar clinician on the types of questions to ask.
Best-corrected visual acuity should be measured via traditional means. Dynamic visual acuity should follow to compare visual acuity while the head is in motion relative to the static baseline acuity. This assessment probes the ability of the vestibular ocular reflex (VOR) to maintain stable vision during head movement, known as gaze stabilization.
To perform, use the best corrected visual acuity as the smallest line, and present 4 lines of acuity above it. If best-corrected acuity is 20/20, the Snellen chart should show 20/50 progressing down to 20/20. While viewing the chart, ask the patient to move their head side to side (approximately 20˚ left and right of midline) at a frequency of 2 complete side-to-side cycles per second and to read to the lowest line that they can. A reduction in acuity to 2 lines worse than baseline indicates a defect in the responsiveness of the VOR.
Computerized visual fields should be performed to rule out ischemic or compressive events along the traditional sight pathway. A careful anterior and posterior segment evaluation will reassure the clinician that all visual complaints are related to visual cortical phenomenon.
To measure binocular function, start with a cover test at distance and near. While the patient is viewing the target with good posture and head position, ask the patient if the target moves side to side, up and down, or diagonal. This can clue the clinician to identify slight vertical heterophoria that can cause significant symptoms.
While performing a pursuit and saccade assessment, ask the patient if moving the eyes triggers symptoms and note hypermetric or hypometric function.
In addition to a traditional near-point of convergence (NPC) assessment, a red lens NPC test can pick up subtle deficiencies in sensory fusion. To perform, ask the patient to put on red/green glasses over their habitual near correction and use a penlight. Starting outside the range of dissociation noted on the traditional NPC, move the penlight inward until diplopia is reported, and repeat 3 times. Diplopia at 7 cm or beyond is a failure.
A small study showed that 76% of mTBI sufferers complained of photophobia.6 Evidence also shows that mTBI sufferers have a lower critical flicker frequency,7 indicating this patient population is more sensitive to the frequency at which an intermittent light stimulus appears to be completely steady. Therefore, due to the amount of screen time in this era, mTBI patients often are more comfortable wearing the highest level of ophthalmic grade blue-blocking lenses. These are a readily available tool in the primary care clinician’s arsenal that can provide dramatic relief.
Refer for therapy
Approximately 28 days or more after injury, the presence of reduced dynamic visual acuity, a cover test outside clinical norms and/or with the presence of a vertical heterophoria, symptoms during eye movements, and/or reduced NPC indicate a referral to a colleague who can offer optometric vision therapy.
Working with patients who have suffered from TBI is a rewarding aspect of optometric care. For more information on how to work with this patient population, visit https://visionhelp.com/concussion/ or www.noravisionrehab.com.
References
1. TBI: get the facts. CDC. Updated March 11, 2019. Accessed February 28, 2021. https://www.cdc.gov/traumaticbraininjury/ get_the_facts.html
2. Goodrich GL, Kirby J, Cockerham G, et al. Visual function in patients of a polytrauma rehabilitation center: a descriptive study. J Rehabil Res Dev. 2007;44(7):929-36. doi: 10.1682/ jrrd.2007.01.0003
3. Eye and vision care followomg blast exposure and/or possible traumatic brain injury. Vision Center of Excellence. Updated 24 November 2015. Accessed February 28, 2021. https://vce. health.mil/Products/Additional-Resources/TBI-CR-Photo
4. Master CL, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-267. doi: 10.1177/0009922815594367
5. McCrory P, Meeuwisse W, Dvořák J, Divine J. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi:10.1136/ bjsports-2017-097699
6. Clark J, Hasselfeld K, Bigsby K, et al. Colored glasses to mitigate photophobia symptoms posttraumatic brain injury. J Athl Train. 2017;52(8): 725-729. doi: 10.4085/1062-6050-52.4.04
7. Chang TTL, Ciuffreda KJ, Kapoor N. Critical flicker frequency and related symptoms in mild traumatic brain injury. Brain Inj. 2007;21(10):1055-1062. doi: 10.1080/02699050701591437

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.