Why doctors are rethinking AMD standards

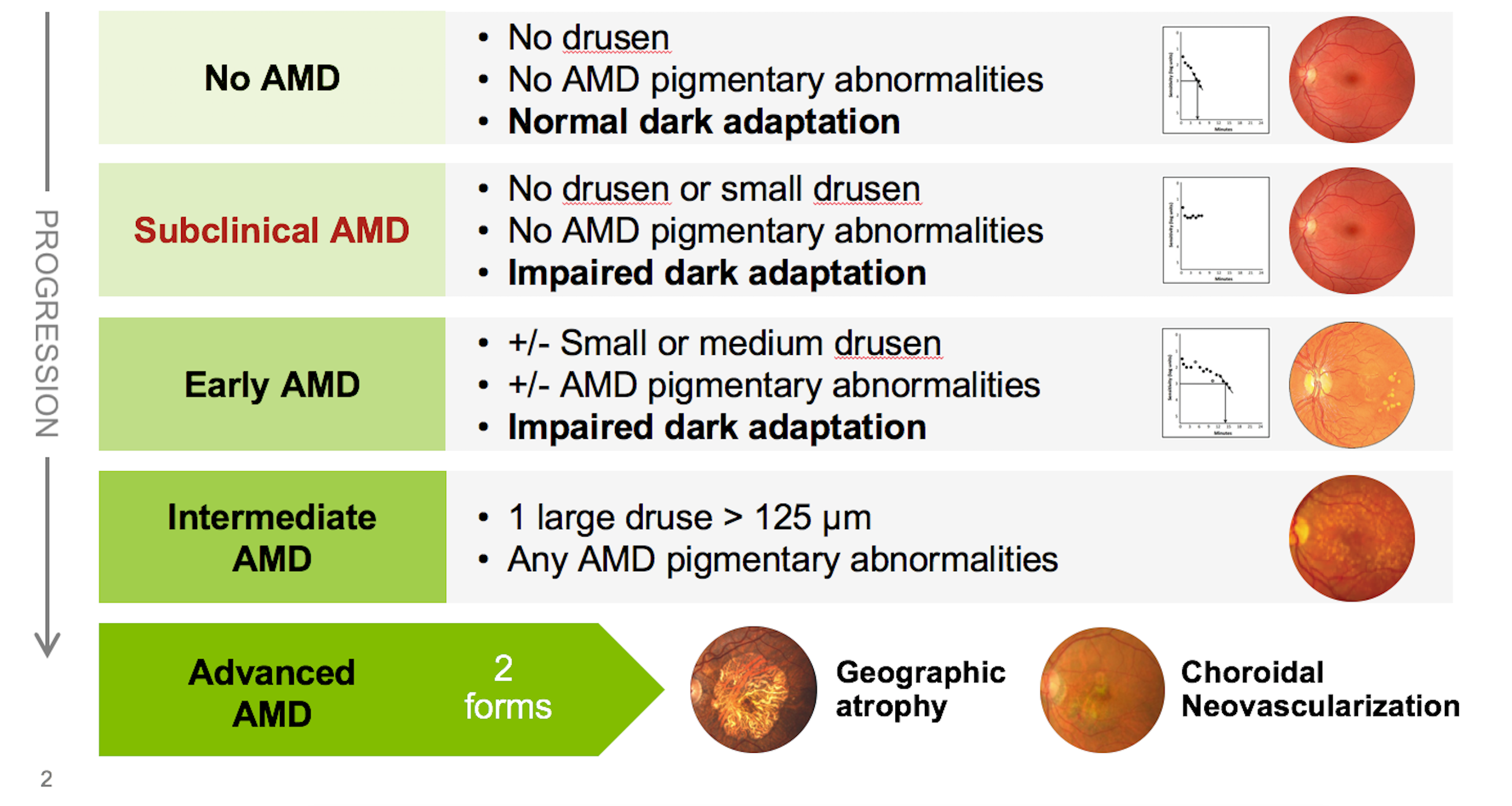
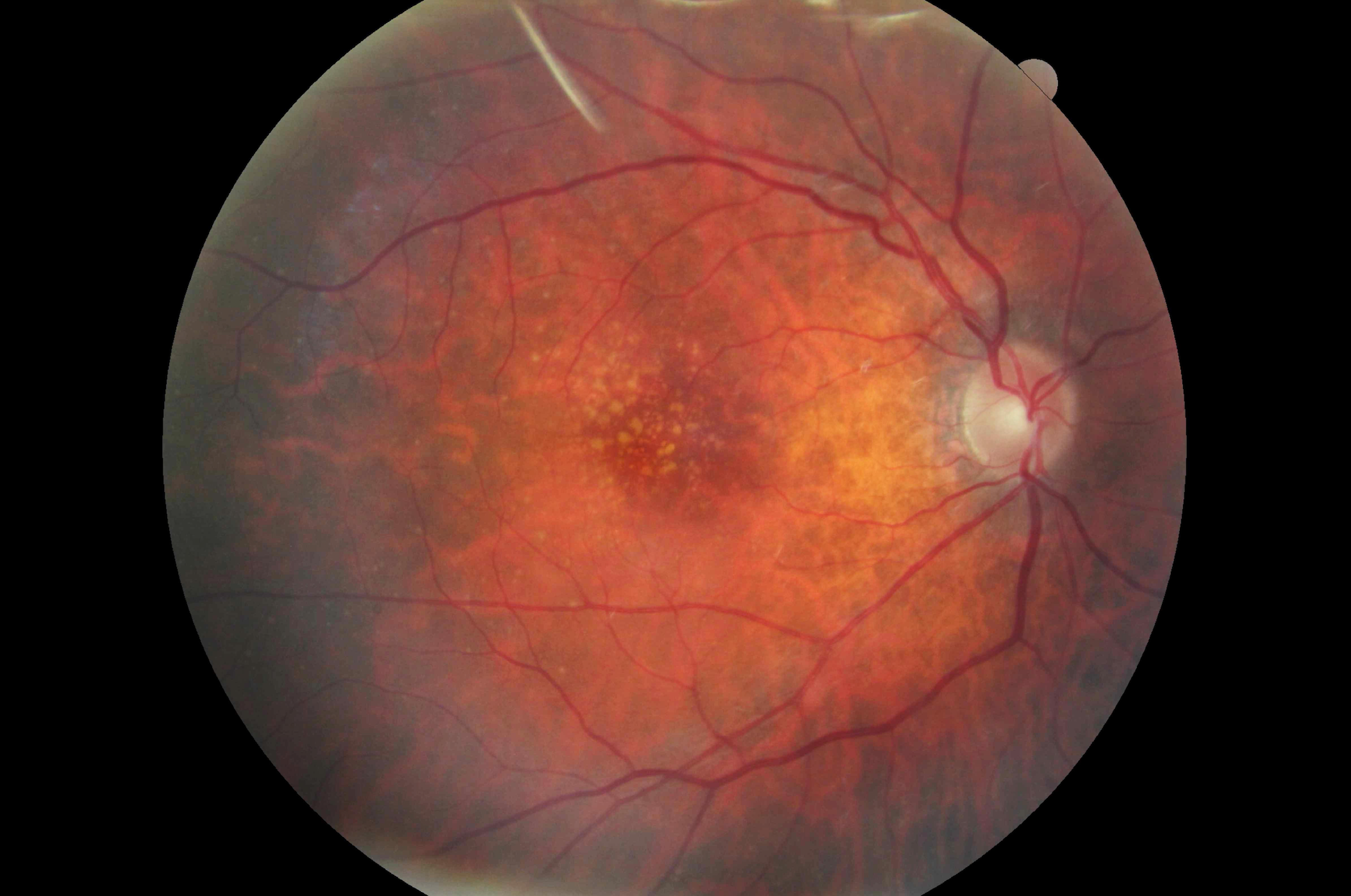
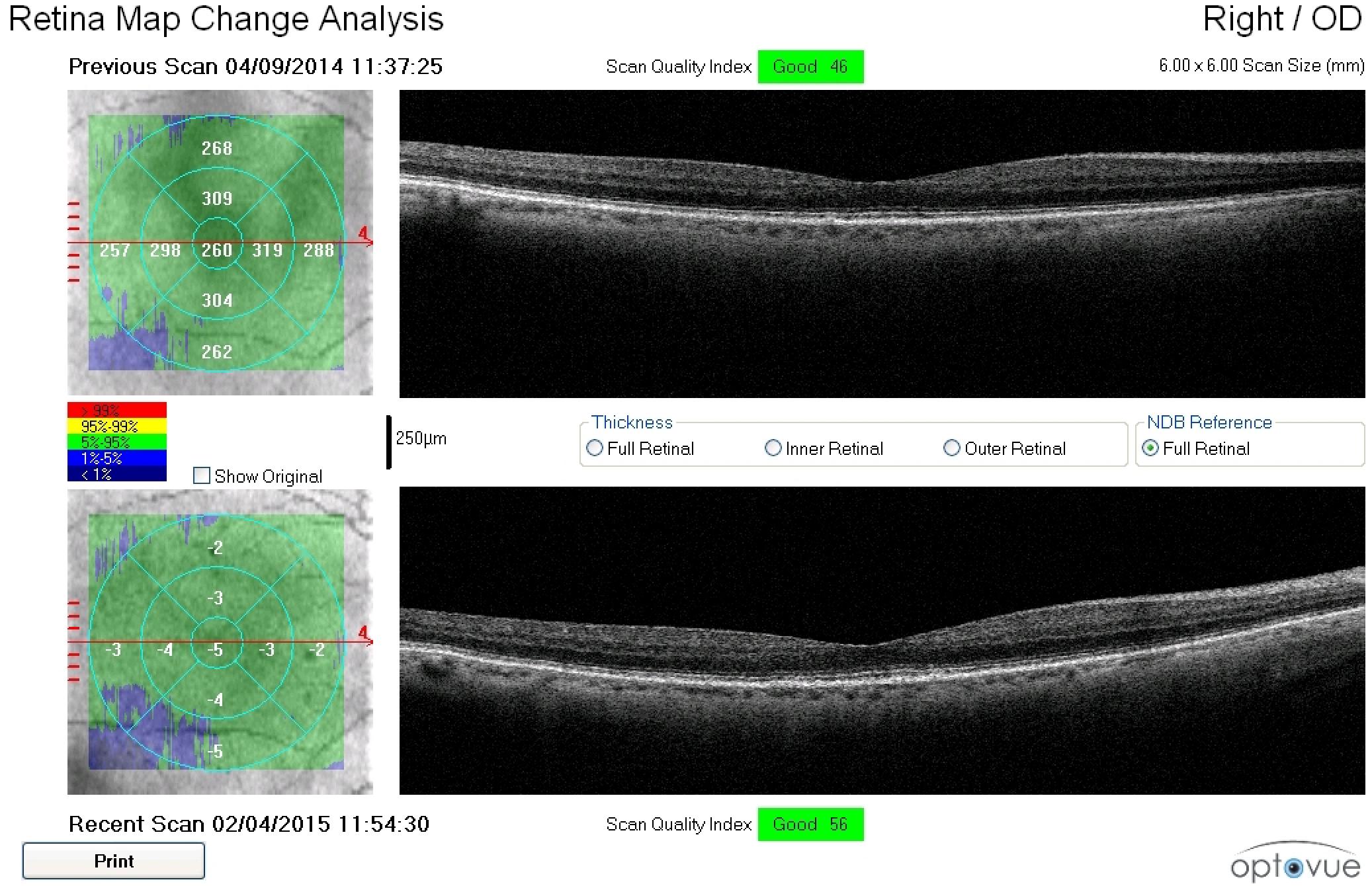
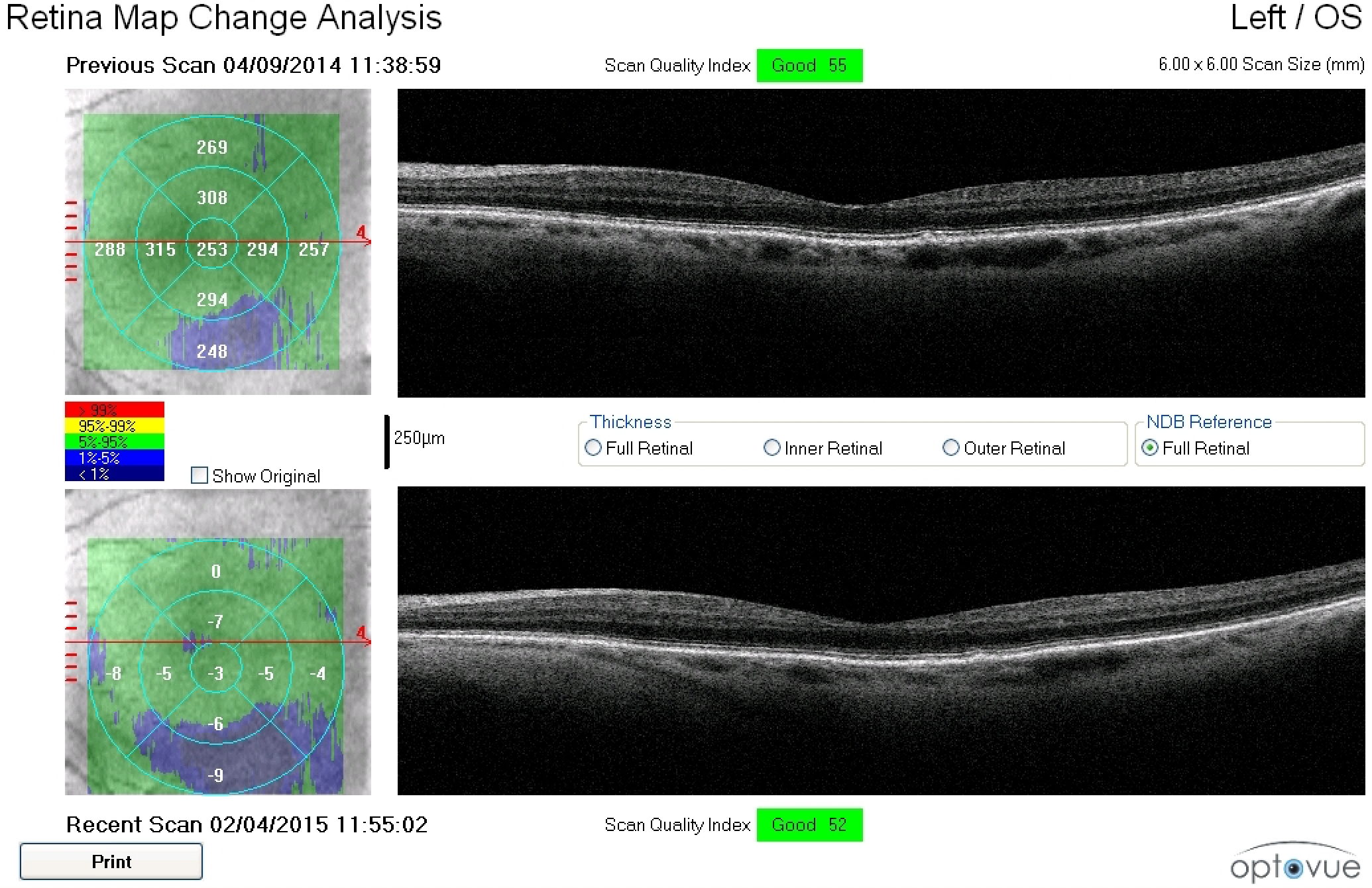
Figure 2. A 79-year-old white female with a family history of macular degeneration in her maternal grandmother presented for an exam. Dilated examination revealed many small mixed hard and soft drusen centrally OD and cluster of small hard drusen in the temporal macula OS. Because of this, dark adaptometry and OCT imaging was ordered. Photos courtesy Amanda Legge, OD
A group of educators and private practice clinicians developed practical, evidence-based guidelines that can be implemented in any eyecare practice to allow for better disease detection.
Beyond grading systems
When asked to explain the current standards in age-related macular degeneration (AMD) treatment and management, most primary-care optometrists will likely recite details of a grading system developed for epidemiological studies and clinical trials. These grading systems rely on careful inspection of three-field stereo-fundus photographs to grade the presence of drusen and/or pigmentary changes.
For this and other reasons, these systems have not been-and are not likely to be-widely adopted in primary eye care. It is also worth noting that as many as 78 percent of patients are first diagnosed with AMD having already suffered irreversible vision loss, and nearly half of them are first diagnosed with an acuity of 20/200 or worse.1,2
Wake-up call
There is no doubt that many AMD patients are overlooked. A study published in JAMA Ophthalmology showed just how often a diagnosis is missed.3
This cross-sectional study, which included 1,288 eyes (644 adults) from patients enrolled in the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR),4,5 revealed that doctors are missing AMD about 25 percent of the time.
Also quite concerning is that 30 percent of the undiagnosed eyes in the study had large drusen, a known risk factor for wet AMD.3
The authors set out to determine to what extent AMD is underdiagnosed by optometrists and ophthalmologists when the disease is actually present. In the study, they reviewed the medical records of 644 adults 60 years or older who were enrolled in ALSTAR.
To be eligible, the patient’s medical record from the most recent comprehensive dilated examination did not indicate a diagnosis of AMD in either eye, and the medical record notes did not contain terms that signified the signs of AMD.
Each patient in the ALSTAR study had digital color fundus photos taken, which were reviewed by masked, trained graders who determined the presence or absence of AMD findings according to the Clinical Age-Related Maculopathy Staging (CARMS) system.6 The types of AMD-associated lesions also were noted.
Results revealed that one of four eyes studied was not diagnosed with AMD during the dilated fundus examination, despite these eyes having macular characteristics indicative of AMD in the fundus photos. Approximately three-fourths of the 320 undiagnosed eyes had 10 or more small drusen (249 [77.8 percent]) and/or intermediate drusen (250 [78.1 percent]), and 96 (30.0 percent) of the undiagnosed eyes had large drusen.3
Preserve visual function
The goal of managing AMD is to preserve visual function. To achieve this goal, proper early detection, diagnosis, monitoring, and treatment must be practiced.
Unfortunately, many doctors are passive when diagnosing and treating nonexudative AMD.1,7 Nonexudative AMD is often not diagnosed until the patient presents with drusen and visual acuity loss. By this criterion, the patient likely has had the disease for years. The patient has lost some of the potential benefits of treatment. In addition, the patient is at higher risk of central visual loss, especially in the first eye that progresses to choroidal neovascularization (CNV).8,9
There is no cure for AMD, so eyecare professionals must try harder to halt or slow the disease progression. Earlier detection allows for earlier treatment, which can potentially lead to better patient outcomes. With proper care, significant visual acuity loss may be prevented in many patients. In fact, authors of the JAMA study point out that improved AMD detection strategies may be needed because many of the patients with missed diagnoses would have been candidates for therapeutic intervention with nutritional supplements.3
Simplified staging
The Beckman Initiative for Macular Research published a classification system designed for use in a primary care setting.10 The practical guidelines presented here are a modification of the Beckman system (see Figure 1).
However, the Beckman system is based solely upon structural findings-whereas the practical guidelines are based upon the structural findings and augmented by functional findings. As with other diseases, such as glaucoma, diagnosing and staging AMD is most accurately and easily achieved when both structure and function are taken into consideration.
Another key difference between the new practical guidelines and the Beckman system is that the new system defines a disease stage called “subclinical AMD,” which is the stage at which abnormal dark adaptation is present in the absence of drusen and/or RPE pigmentary changes.
In AMD, functional deficits often exist before structural change is evident. Histopathological studies have shown that retinal pigment epithelium (RPE) cells deposit locally generated cholesterol beneath the RPE cell layer and in Bruch’s membrane before drusen are formed.11
With disease progression, cholesterol continues to accumulate, resulting in focal areas that are sufficiently thickened to be identified as drusen. Thus, drusen caused by AMD are the tip of an iceberg of the earliest lesions caused by AMD. More dysfunction is present than would be concluded simply on the appearance of drusen.
The fact that a patient has visual symptoms of impaired dark adaptation before we can clinically detect AMD illustrates the imperative to be more proactive in early detection and intervention. These functional deficits are easily detected using dark adaptation testing. Indeed, impaired dark adaptation, which is often expressed by the patient as night vision difficulties, is often the first detectable consequence of AMD and can be used to identify patients with subclinical disease.
References:
1. Olsen TW, Feng X, Kasper TJ, Rath PP, Steuer ER. Fluorescein angiographic lesion type frequency in neovascular age-related macular degeneration. Ophthalmology. 2004 Feb;111(2):250-255.
2. Cervantes-Castañeda RA, Banin E, Hemo I, Shpigel M, Averbukh E, Chowers I. Lack of benefit of early awareness to age-related macular degeneration. Eye (Lond). 2008 Jun;22(6):777-781.
3. Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G, Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017 Jun 1;135(6):570-5.
4. Owsley C, Huisingh C, Jackson GR, Curcio CA, Szalai AJ, Dashti N, Clark M, Rookard K, McCrory MA, Wright TT, Callahan MA, Kline LB, Witherspoon CD, McGwin G Jr. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014 May 22;55(8):4776-89.
5. Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G Jr. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res. 2016;41(2):266-72.
6. Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006 Feb;113(2):260-6.
7. Chevreaud O, Semoun O, Blanco-Garavito R, Kamami-Levy C, Merle B, Jung C, Querques G, Souied EH. Visual acuity at presentation in the second eye versus first eye in patients with exudative age-related macular degeneration. Eur J Ophthalmol. 2016 Jan-Feb;26(1):44-47.
8. Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR; MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007 Feb;114(2):246-252.
9. Loewenstein A, Richard & Hinda Rosenthal Foundation. The significance of early detection of age-related macular degeneration: Richard & Hinda Rosenthal Foundation lecture, The Macula Society 29th annual meeting. Retina. 2007 Sep;27(7):873–878.
10. Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013 Apr;120(4):844-851.
11. Owsley C, McGwin G, Jackson GR, Heimburger DC, Piyathilake CJ, Klein R, White MF, Kallies K. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006 Apr;47(4):1310-1318.
12. Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001 Apr;108(4):697-704.
13. Caban-Martinez AJ, Davila EP, Lam BL, Dubovy SR, McCollister KE, Fleming LE, Zheng DD, Lee DJ. Age-related macular degeneration and smoking cessation advice by eye care providers: a pilot study. Prev Chronic Dis. 2011 Nov;8(6):A147.
14.Hobbs RP, Bernstein PS. Nutrient supplementation for age-related macular degeneration, cataract, and dry eye. J Ophthalmic Vis Res. 2014 Oct-Dec;9(4):487-493.
15. Carneiro Ã, Andrade JP. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxid Med Cell Longev. 2017;2017:6469138.
16. Mares JA, Voland RP, Sondel SA, Millen AE, Larowe T, Moeller SM, Klein ML, Blodi BA, Chappell RJ, Tinker L, Ritenbaugh C, Gehrs KM, Sarto GE, Johnson E, Snodderly DM, Wallace RB. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol. 2011 Apr;129(4):470.
17. McGuinness MB, Le J, Mitchell P, Gopinath B, Cerin E, Saksens NTM, Schick T, Hoyng CB, Guymer RH, Finger RP. Physical activity and age-related macular degeneration: a systematic literature review and meta-analysis. Am J Ophthalmol. 2017 Aug;180:29-38.
18. Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist–hip ratio. Arch Ophthalmol. 2003 Jun;121(6):785-92.
19. Choudhury F, Varma R, McKean-Cowdin R, Klein R, Azen SP; Los Angeles Latino Eye Study Group. Risk factors for four-year incidence and progression of age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2011 Sep;152(3):385-395.
20. Sui G-Y, Liu G-C, Liu G-Y, Gao YY, Deng Y, Wang WY, Tong SH, Wang L. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013 Apr;97(4):389-394.
21. AAO Retina/Vitreous PPP Panel, Hoskins Center for Quality Eye Care. Age-Related Macular Degeneration PPP - Updated 2015. American Academy of Ophthalmology. Available at: https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015. Accessed 10/30/18.
22.Owsley C, McGwin G Jr, Clark ME, Jackson GR, Callahan MA, Kline LB, Witherspoon CD, Curcio CA. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016 Feb;123(2):344-51.
23. Owsley C, Jackson GR, White MF, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001 Jul;108(7):1196-202.
24. Curcio CA, Johnson M. Structure, function, and pathology of Bruch’s membrane. In: Ryan SJ, et al, eds. Retina, Vol 1, Part 2: Basic Science and Translation to Therapy. 5th ed. London: Elsevier; 2013:466-81.
25.Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmo Vis Sci. 2014 Mar 10;55(3):1427-31.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.