Central serous retinopathy is not a benign disease
Remain vigilant to recurrences to apply potential treatments

Words to this effect were in a referral letter from a retinologist more than 30 years ago for a patient presenting with acute blurry vision: “Central serous retinopathy is not a benign disease.”
The patient's vision in the involved eye at that presentation was 20/40, and the diagnosis was central serous retinopathy (CSR). The patient was observed, and visual acuity resolved to 20/25.
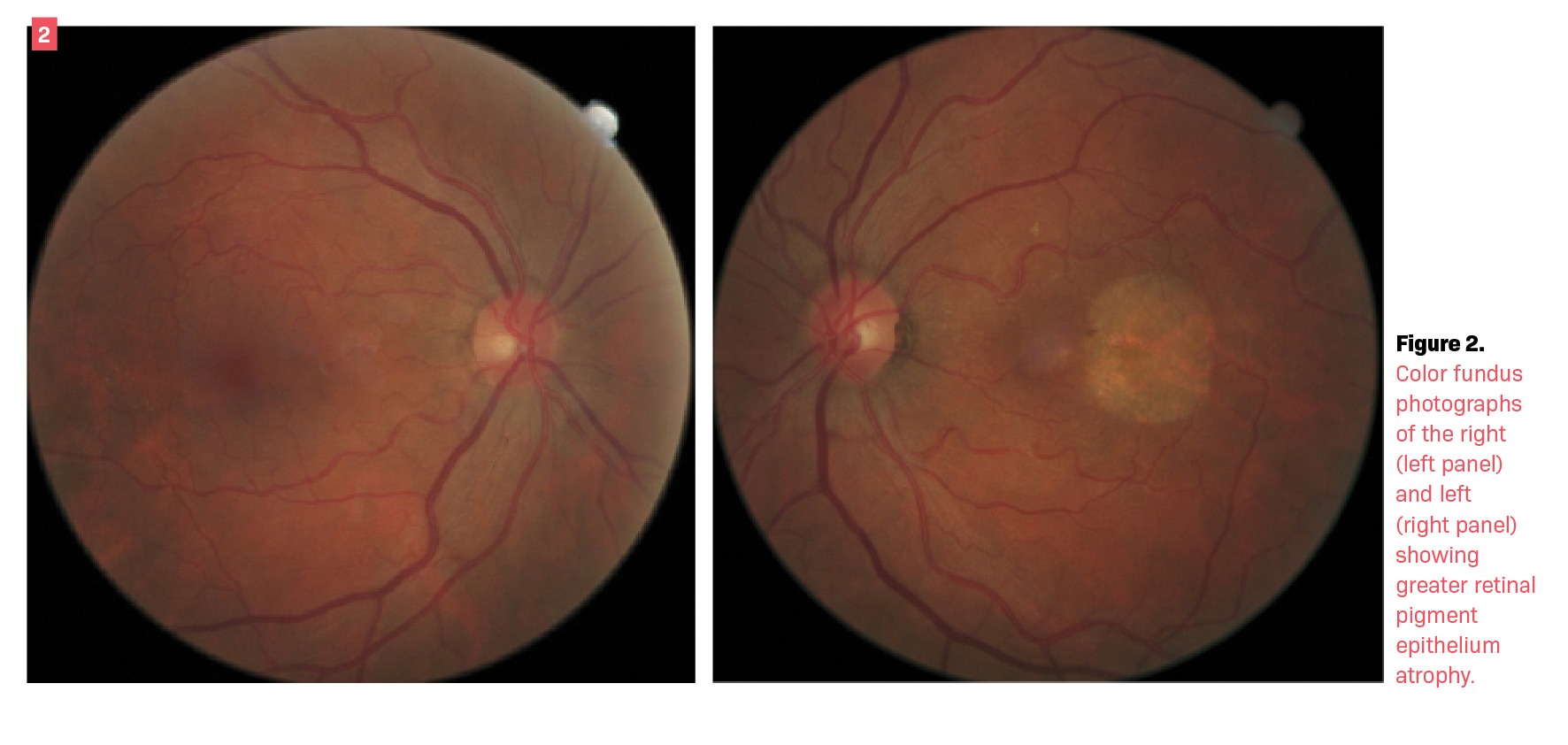
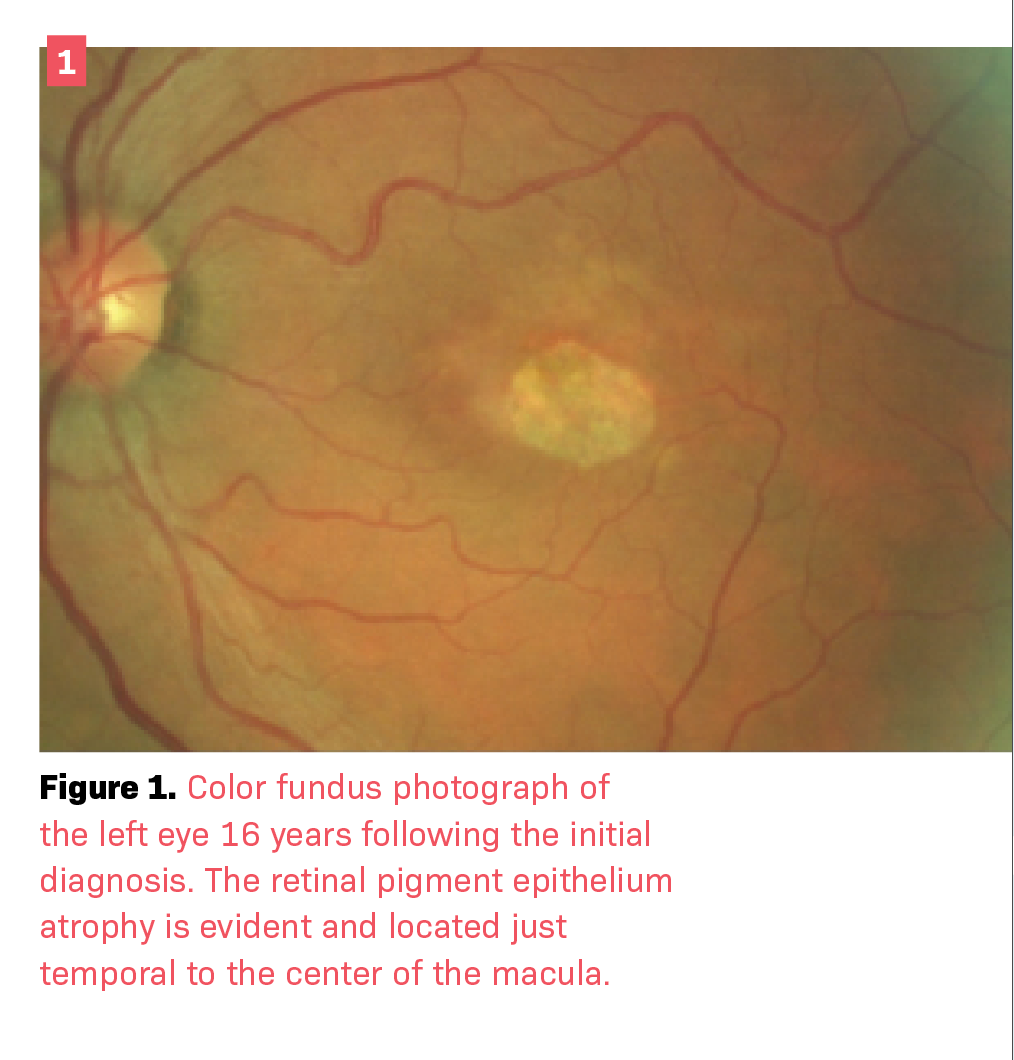
Over decades of follow-up, the patient showed progressive retinal changes without the presence of subretinal fluid (Figures 1 and 2). In the absence of acute leakage into the subretinal space, no treatment was indicated.
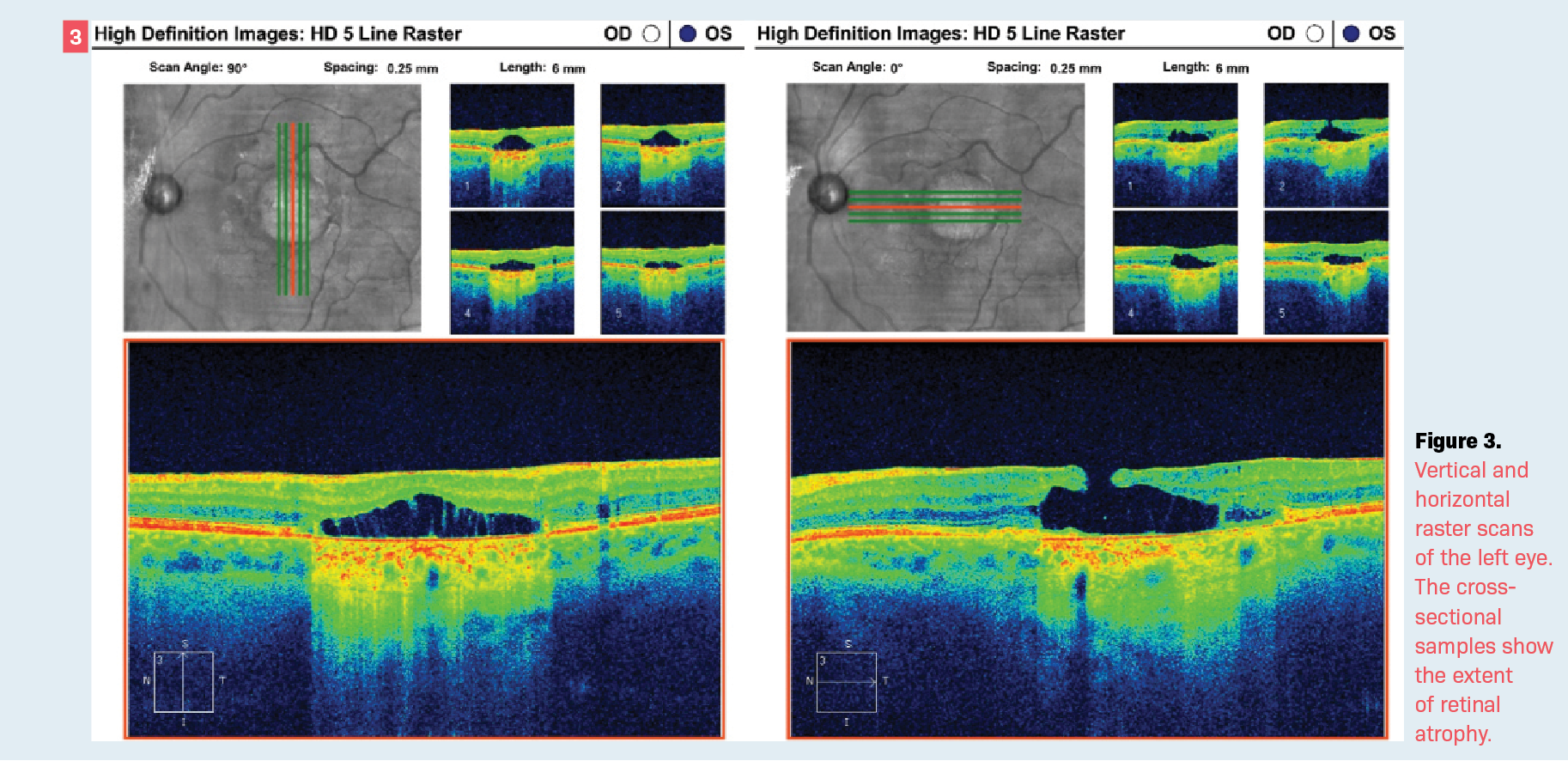
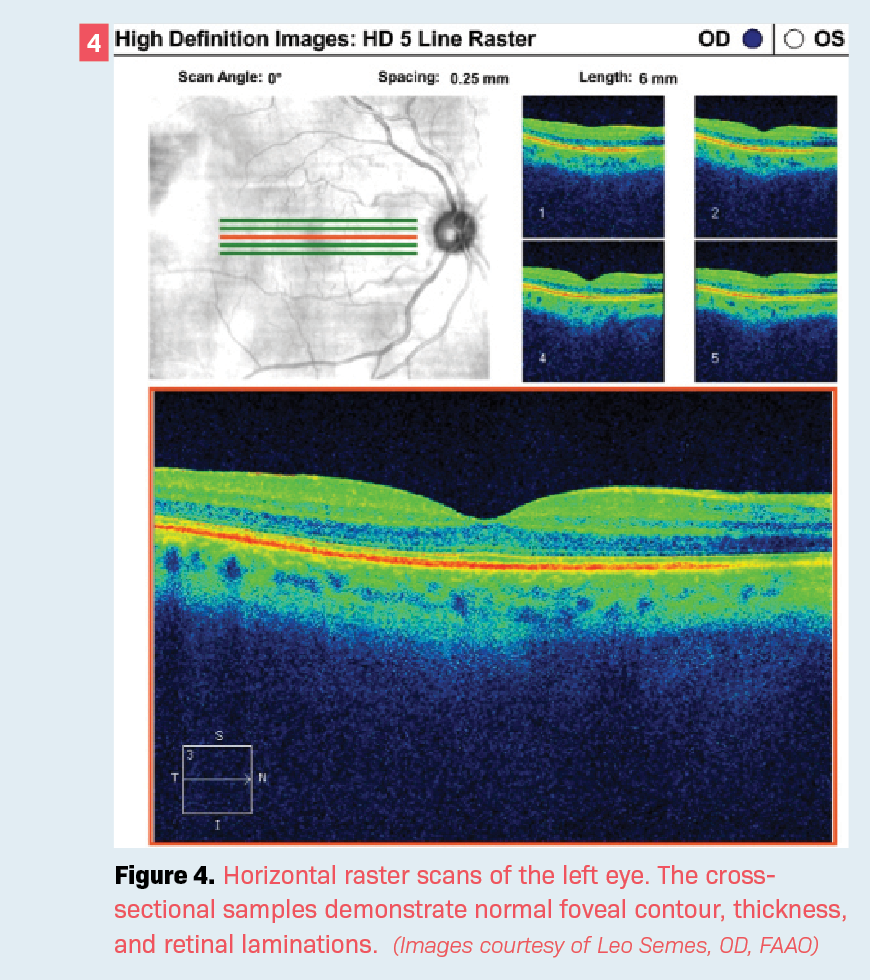
At the most recent follow-up visit, the patient—now aged 68 years—exhibited visual acuity of 20/80 in the involved eye. The visual acuity in the fellow eye (OD) was 20/20. Optical coherence tomography (OCT) shows a full-thickness retinal defect, which allows for differentiation of the choroidal circulation in the involved eye (OS) (Figure 3) while the right eye is completely normal. The OCT imaging of the right eye shows normal retinal laminations and foveal contour (Figure 4).
Discussion
CSR has many appellations, including central serous chorioretinopathy, perhaps the best clinical description because it incorporates the pathogenesis of choroidal hyperperfusion. The prevalence of the presentation has caused some eye care clinicians to simply use the term central serous.
A recent review on the subject describes the condition as common among those aged 25 to 50 years with a predilection for men. Although the prognosis for spontaneous return to baseline visual acuity in acute cases is expected, a variety of treatment interventions have been reported as successful for chronic cases.1 In the present case, no triggers were identified but corticosteroid therapy has been reported, which can be modified or avoided to minimize the possibility of recurrences.2
Practitioners should remain vigilant, especially with patients with chronic disease, so that they are aware of recurrences for which potential treatments may be applied. It is beyond the scope of this discussion to offer all those options. The interested reader is referred to publication on the subject.2-5 One potential therapy applicable to primary eye care is eplerenone, the oral medication that appears to be promising.6 The author has had limited success with topical nepafenac (unpublished results).
Interestingly, patients with chronic cases may develop neovascularization that may be the cause of retinal complications.7 Fortunately, the present case did not exhibit evidence of this complication. We are unable to determine the cause of the unfortunate outcome.
References
1. Semeraro F, Morescalchi F, Russo A, et al. Central serous chorioretinopathy: pathogenesis and management. Clin Ophthalmol. 2019;13:2341-2352. doi:10.2147/OPTH.S220845
2. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systemic review and meta-analysis. Retina. 2016;36(1):9-19. doi:10.1097/IAE.00 00000000000837
3. van Rijssen TJ, van Dijk EHC, Yzer S, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:100770. doi:10.1016/j.preteyeres.2019.07.003
4. Sartini F, Figus M, Nardi M, Casini G, Posarelli C. Non-resolving, recurrent and chronic central serous chorioretinopathy: available treatment options. Eye (Lond). 2019;33(7):1035-1043. doi:10.1038/s41433-019-0381-7
5. van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547-1555. doi:10.1016/j.ophtha.2018.04.021
6. Fusi-Rubiano W, Saedon H, Patel V, Yang YC. Oral medications for central serous chorioretinopathy: a literature review. Eye (Lond). 2020;34(5):809-824. doi:10.1038/s41433-019-0568-y
7. Aggarwal VK, Agarwal P, Azad S. Retinal neovascularization in a patient with chronic central serous chorioretinopathy. Indian J Ophthalmol. 2020;68(8):1693-1695. doi:10.4103/ijo.IJO_2068_19

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.