How inflammation may play a role in retinal disease
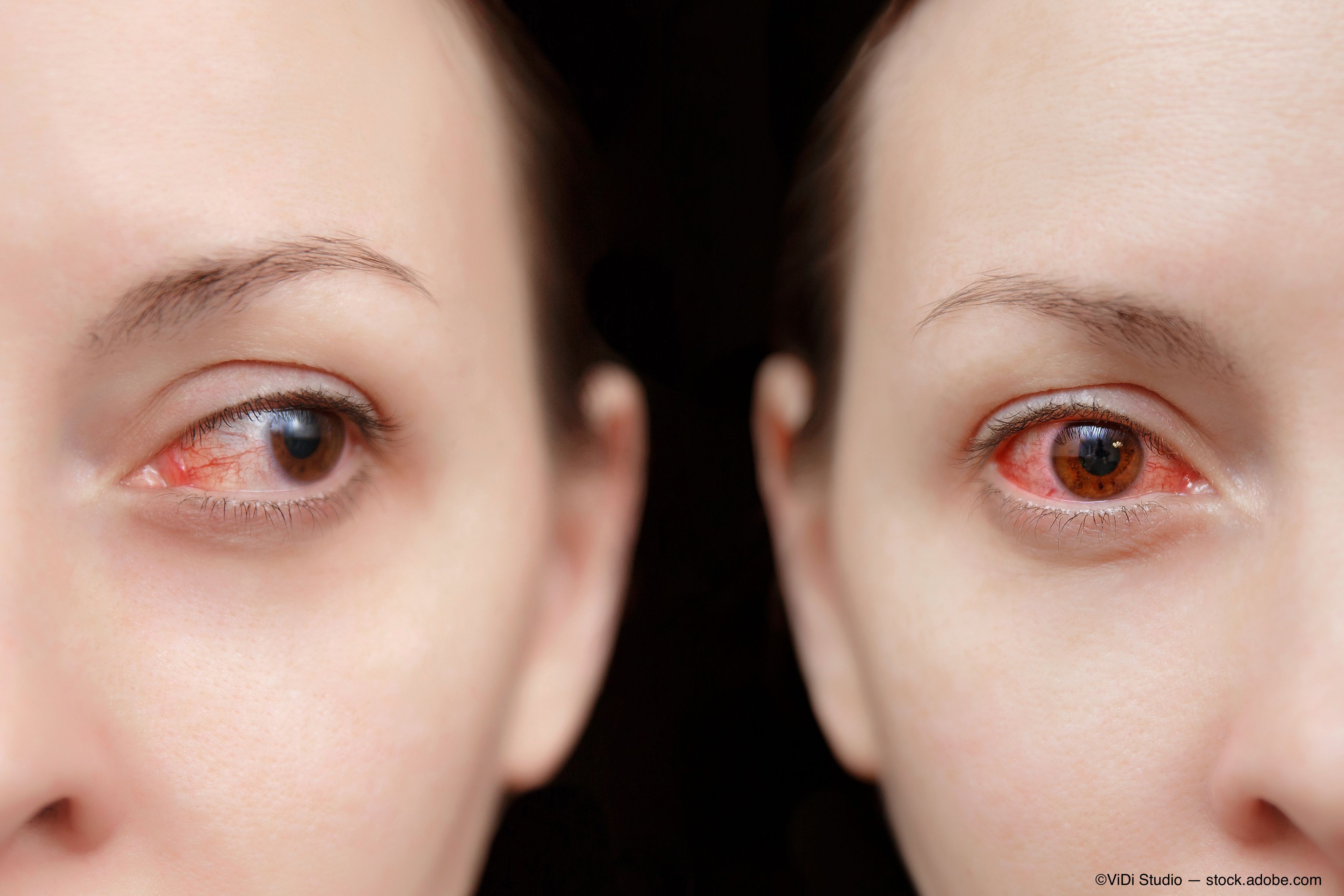

Whenever I deem a case of viral conjunctivitis to be significant enough to warrant the prescription of a topical steroid, I have a very brief discussion with the patient beforehand.
I explain that I’m not targeting the virus itself, but rather the patient’s immune response to the viral insult. I liken it to a cold in which supportive therapy and anti-inflammatory medications don’t kill the virus but rather make the patient feel better as the condition runs its course.
It’s possible that similar conversations may be had in the future with respect to common retinal diseases as we become more familiar with the underlying mechanisms of action (however complex and multifactorial they may be).
Previously by Dr. Casella: Ensure patients know follow-up visits may be covered
Retinal immune response
An August 2019 review published online in Frontiers in Immunology does well to lay out where we stand with respect to our understanding of the link between the immune system and the various structures and chemicals that make up the neurosensory retina.1
This paper focuses specifically on microglia, the macrophages of the retina whose function is to go around eating up undesirable particles.
In addition to giving a nice synopsis of the history and discovery of microglia in the nervous system, the authors explain what we know about the role of these agents with respect to the formation and progression of several common degenerative retinal conditions. Additionally, the role of microglia during retinal development and subsequent retinal function is discussed.
Age-related macular degeneration (AMD) is discussed in detail with regard to the immune system. Complement factors and other inflammatory proteins have been found in elevated levels in the presence of AMD.
Enlarged microglia have been isolated in close proximity to retinal drusen, as drusen are chemoattractants for microglia, thus yielding an explanation for their movement into the subretinal space in those with AMD. This provides further evidence for a dysregulated immune response as having a causal role in AMD.
Related: Why doctors are rethinking AMD standards
As the most common hereditary retinal degeneration, retinitis pigmentosa (RP) is also discussed in detail with respect to the immune system’s response as an exacerbating factor.
Microglia have been found to become enlarged with the cellular debris of diseased rods in RP, and these enlarged microglia have been shown in animal models to secrete pro-inflammatory proteins which are neurotoxic to adjacent unaffected retinal cells.
As for diabetic retinopathy, enlarged ameboid-shaped microglia have been shown to be present at all stages of this common cause of blindness.
Related: Case report: Don't be misled by diabetic retinopathy
In the early non-proliferative form, these cells are seen in elevated numbers around retinal hemorrhages and microaneurysms. As the disease progressed, so does their presence, which is especially high in the vicinity of cotton-wool spots and engorged blood vessels. Finally, in proliferative diabetic retinopathy, these enlarged microglia are found in high quantities around areas of neovascularization-both at the optic nerve head and the nerve fiber layer of the retina.
Why is this important? It is important because these reactive cells-through their communication with inflammatory molecules-are thought to have an association with the increase in vascular permeability seen in diabetic retinopathy.
Glaucoma in the mix
With respect to glaucoma, the authors do well to point out the fact that “to date, the only proven therapeutic approach to prevent development and slow down disease progression is the lowering of intraocular pressure.”
However, insult to the optic nerve can persist in spite of and in the presence of so-called “normal” intraocular pressure. You can tell where this is going… There exists a neuroinflammatory process in glaucoma (yes, microglia play a role).
As glaucoma progresses, these enlarged and reactive microglia (which serve to dispose of unwelcome cells and molecules) seem to play a role in furthering the premature death of the cells that converge to form the optic nerve head.
Related: Consider IOP fluctuations when diagnosing glaucoma
What’s next
The authors of this paper do well to point out the fact that we still do not have an adequate understanding of the process by which retinal microglia become overly engorged and overly reactive in the presence of retinal disease.
However, a more comprehensive understanding of such may lead to a pharmacological future in which we make use of targeted immunosuppressive therapies which address this very concern-overall blocking of microglial expression would do more harm than good because these cells clearly serve a purpose in normal healthy retinas in addition to healthy portions of diseased retinas.
This paper is deserving of your time and certainly worth the read. As a clinician, I found myself happy to get down into the weeds of this paper and came out with a more comprehensive understanding of where we stand with respect to this concept.
Read more by Dr. Casella
References:
1. Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in Retinal Degeneration. Front Immunol. 2019 Aug 20;10:1975. doi: 10.3389/fimmu.2019.01975. eCollection 2019.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.