Overlooked causes of dry eye
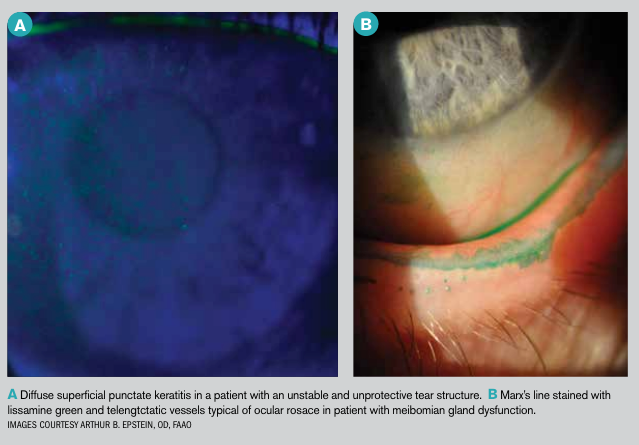

With an explosion in the use of digital devices, shifts in diet and other likely contributing factors, eyecare providers are encountering a veritable epidemic of “dry eye.”
Every day, a growing number of patients present complain of dry, gritty, uncomfortable eyes, blurred vision and chronic eye redness.
Despite “dry eye” being increasingly recognized as a complex, chronic, and progressive condition, and all of the attention being paid to the disorder, many patients still leave their eyecare providers’ offices with a sample of artificial tears despite having tried and failed with similar products on their own. This creates a cycle of frustration for both patient and practitioner.
Related: The evolution of tear substitutes
More persistent patients sometimes return, often with the exact same complaints. Those more frustrated or symptomatic may seek another opinion from another doctor, usually with the same result. More wetting drops, an Rx drug approved to make more tears, or punctal plugs to slow tear drainage usually follow.
For most, relief is fleeting at best, suffering worsens, and the cycle continues its downward spiral.
Patients begin to feel increasingly hopeless and doctors become frustrated when their treatments fail. While there are many reasons for treatment failure, perhaps the most common is that most eyecare providers persist in treating symptoms rather than the actual causes of the disease.
This article explores the underlying causes of dry eye and the contributing elements that are often missed in its diagnosis and treatment. My approach explores elements of ocular surface design and related human engineering based on the likely original functional intent of system elements.
Related: How to incorporate nutrition into dry eye practice Complexity of the ocular surface
The tears seem simple enough. Most patients encounter the streams of salty water coursing down their cheeks early in life and don’t realize that there is more than one type of tears.
Reflex tears are what most think of as tears; however, reflex tears function primarily as built-in natural emergency eyewash. They are different from the more complex and structured basal tears that maintain vision and ocular surface protection. It is important that patients understand the distinction.
Viewing tears as simple belies the complexity of tear composition, structure, function, and how tears interact with the ocular surface. The tears and external eye coexist to provide a smooth and stable refractive surface overlying the relatively irregular corneal epithelium.
Without this stable tear structure, vision would be limited. Especially in a primal environment, early humans may have fallen prey to predators or, being unable to successfully forage for food, starved.
In essence, the tears, the ocular surface and the systems that support their interaction are critical parts of an essential survival strategy. A stable tear film and acute vision may be among reasons why man has thrived.
Such an originalist view of the ocular surface is key to understanding both function and dysfunction of the tears and ocular surface.
Related: ODs reach for artificial tears first to treat dry eye
In 2017, the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II defined dry eye as a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1
Although DEWS II offers the most comprehensive view of dry eye to date, its complexity can be daunting for many clinicians, especially in diagnosing and managing the condition.
Perhaps the most powerful part of the current definition is the reference to the loss of homeostasis of the tear film. Because the eye is so critical to survival, much of its function centers around maintaining or, when necessary, regaining the physiologic balance necessary for sustained stable vision, such as protecting and repairing the ocular surface when damage or disruption occurs.
Baudouin, in a seminal treatise on dry eye, approached the disorder combining a comprehensive and holistic perspective.2 His vicious cycle underscores the complexity and multifactorial nature of what we call dry eye and identifies key elements that are root causes as well as contributing factors. Some are not well recognized.
As mentioned previously, in understanding tear dysfunction, comprehending the original intent of the systems that support structure and function is critical. Baudouin’s approach is particularly valuable for clinicians seeking ways to interrupt the ongoing down-spiral of dry eye.
Do patients actually have dry eyes?
The term “dry eye” remains in common use despite being a poor description of the disease. A truly dry eye would be opaque and dysfunctional. Thankfully, most “dry eyes” remain reasonably functional.
Related: How to treat dry eye in the pediatric and young adult population
In 2006, the Delphi panel suggested the use of the term “tear dysfunction” rather than “dry eye.”3 Korb and Blackie described dry eye in 2015 as the wrong diagnosis for millions.4
In a practice limited to dry eye in Phoenix, I rarely, if ever, see purely aqueous deficient dry eye. That includes patients suffering from Sjögren’s syndrome and other immune disorders usually associated with aqueous deficiency. The majority of these patients have meibomian gland dysfunction (MGD) and other contributing factors.
In my experience, it seems unlikely that aqueous deficient and evaporative dry eye are able to exist in complete isolation.
So, why do eyecare practitioners persist in using the term “dry eye”?
Related: Blog: Why dry eye?
Until recently, dryness due to aqueous deficiency has been accepted as the primary cause of most dry eye. This simplistic perspective dates back to the Ebers Papyrus.5
In their writings, the ancients appeared to be fascinated with tears and associated their absence with a variety of eye problems.
Even throughout the 1930s and ‘40s, a relative period of dry eye and ocular surface enlightenment, when Sjögren first described his namesake disease5 and ocular anatomist and author Eugene Wolff7 wrote of a three-layer complex tear structure, most of the focus on the pathogenesis of dry eye remained on tear production rather than tear function.
The continued rigid differentiation of dry eye into aqueous deficient or evaporative subtypes adds to the confusion. Beyond all forms of “dry eye” existing on a clinical and interrelated continuum, what eyecare practitioners usually define as aqueous deficient dry eye is often at least partially caused or worsened by evaporation rather than simply by a lack of aqueous production.8,9Related: Blog: Don't leave dry eye disease patients high and dry
What is described as evaporative dry eye is more complex than simple evaporation and often a result of tear instability caused by a combination of meibomian gland dysfunction and enzymatic degradation of tear lipids due to lid bacteria overpopulation associated with MGD.
Both insufficient aqueous and tear lipids result in tear instability and the compromised function and protection that patients experience as signs and symptoms of “dry eye.”10
So, for the majority of dry eye patients, their problem is not a lack of tears but rather dysfunction of the tears they do have.
Related: The most important objective dry eye test is TBUT with fluorescein, ODs say Real causes of tear dysfunction
Effectively managing tear dysfunction requires an understanding of cause and effect.
The effects of dry eye are familiar to both patients and clinicians. They include the usual signs and symptoms like dryness, grittiness, foreign body sensation, red irritated eyes, unstable vision, and corneal and conjunctival staining. The causes are far more complex and potentially confusing to the clinician.
MGD is recognized as both a cause and a contributor to dry eye.8 It is an obstructive and inflammatory disease that results in insufficient and abnormal production of tear lipids. While most associate MGD with excessive tear evaporation, its role in tear dysfunction is far more complicated.
Related: How to start incorporating dry eye in your practice
Meibomian gland lipids are complex and provide a critical element in tear structure.11 Hydrophobic bonding of these lipids creates a tarpaulin-like covering of the ocular surface. Phospholipids in meibum possess both hydrophobic and hydrophilic properties and help bond outer nonpolar lipids to the underlying hydrophilic tear structure.12
Meibomian gland lipids have important tear stabilizing functions beyond serving as an evaporative barrier.13 While eyecare practitioners understand the composition of tear lipids in general terms, the profession is years away from assaying and understanding individual variation and its impact on tear structure and function. This is similar to the evolution of blood analysis.
An unstable tear structure leads to surface exposure and, in more severe cases, frank damage. Maintaining integrity and function of the ocular surface is so critical that the eye is equipped with a variety of mechanisms such as increased mucin production to maintain and regain homeostasis.
Related: New research on improving contact lens comfort for patients with dry eye
Inflammation, which plays an important role in the progression of tear dysfunction, appears to result when balance is not regained, which can lead to even greater dysfunction.2
However, inflammation is a consequence, and with the exception of autoimmune disease, rarely a cause. An unstable tear structure also provides a poor refracting surface, another frequent complaint associated with “dry eye.”
Obstruction is the most widely recognized cause of MGD and resulting tear lipid insufficiency.14 As meibum stagnates and becomes saturated and thickened, pressure within the glands mounts and production is down-regulated.
Related: Blog: Examine evaporative dry eye disease exposure in your patients
In more severe cases, gland tissue is lost through atrophy or as a consequence of localized inflammation as the body attempts to clear rancid lipids within the glands.
Gland clearance has long been recognized as a treatment for MGD.15 Performed appropriately, this includes the application of heat to help melt congealed meibum and mechanical expression.
However, I have found that the regular use of warm compresses and massage, expression of the glands with forceps or paddles, or even the use of advanced technology like automated meibomian gland expression or intense pulsed light (IPL) still results in treatment failure or only temporary relief for some patients.
Based on my experience, understanding and addressing the underlying causes of meibomian gland dysfunction, lipid deficiency, and tear instability can improve patient outcomes dramatically especially when combined with advanced treatment approaches like assisted or automated expression and IPL.
Related: Pros and cons of available MGD treatments Overlooked causes for tear dysfunction
From my perspective, the most overlooked causes and potential cures for tear dysfunction are:
• Changes to the visual environment
• Shifting diet
• Altered lid microbiome and enzymatic degradation of tear lipids
• Lagophthalmos and nocturnal exposure
Changes to the visual environment
The ocular surface and tear film didn’t anticipate Guttenberg and his printing press or Steve Jobs and the iPhone and iPad. Both book reading and computer use result in decreased blink rates and increases in partial blinks.16,17
Because meibum release depends upon normal lid function including full and complete blinking, decreased clearance and stagnation of meibum is a likely consequence of our modern visual environment and excessive digital device use inhibiting normal blink function.
Blinking exercises can be beneficial, but the importance of reestablishing normal blink patterns must be reinforced. Dry eye expert Donald Korb, OD, FAAO, has long been a proponent of blink training and it is an essential part of restoring function to the meibomian glands.
Related: Know the connection between Vitamin D and dry eye
Shifting diet
Think of the meibomian glands as a wood stove. Without wood, no heat would be produced. Likewise, the meibomian glands require essential fatty acids (EFAs) as raw materials to produce the lipids that make up meibum.18
Over the past century, changes in our intake of EFAs have increased the ratio of omega 6 to omega 3 fatty acids.19
Despite the controversy of the recent DREAM study,20 personal experience reinforces the importance of omega 3 supplementation.21
After in-office testing, I currently recommend to patients 2.5 g daily of a re-esterified triglyceride formulation containing a 3:1 EPA to DHA ratio or greater. At least two sources are available: PRN DE which requires four gel caps daily and Nordic Naturals EPA-Xtra which requires three gel caps.
Related: Video: Dry eye company founder talks history, pipeline
Altered lid microbiome and enzymatic degradation of tear lipids
MGD is associated with alterations in the lid microbiome.22 Biofilm accumulation and bacterial overpopulation of the lids with subsequent release of bacterial toxins and elaboration of lipase and other bacterial digestive enzymes occurs.23
Staphylococcal toxins target competing species, but they are pro-inflammatory and cause irritation and inflammation when they get into the eye.
Bacterial elaborated lipase in the tears breaks down and saponifies meibum, producing soaps that cause burning, tear frothing that can be observed on slit-lamp exam (see Figure X), and, more importantly degradation of tear structure and stability.24
This often occurs in the background and is easily missed by the clinician who fails to recognize the importance of the altered microbiome and lid staph overpopulation frequently associated with MGD.2
Management is straightforward and requires the application of hypochlorous directly to the lids and surrounding peri-orbital skin.26 Hypochlorous acid is naturally occurring, produced by neutrophils to counter infection and inflammation.26
It also disrupts bacterial digestive enzymes like lipase and has been used successfully to treat necrotizing fasciitis as well as blepharitis and MGD.27 There are two primary ways to make hypochlorous acid: direct generation and variations of electrophoresis.
I direct patients to spray the hypochlorous acid directly onto their closed eyelids, flutter the lids for a second or two to spread the solution, then keep their eyes closed for 10 seconds before wiping the solution away with a tissue or onto their cheeks if they have rosacea.
In my experience, topical hypochlorous acid appears to reduce skin inflammation in these patients with rosacea.28 I recommend this as part of a morning and evening hygiene routine.
Recently launched HyClear (Contamac) is my preferred hypochlorous acid product. I have found that it is stable; according to the company, it remains active and useable for more than a year after the bottle is opened, has minimal chlorine odor, and is effective clinically, and acts rapidly with most patients experiencing relief within a day or two.
Other available hypochlorous acid products are available from OcuSoft, We Love Eyes, Avenova, and Bruder, among others.
Lagophthalmos and nocturnal exposure
Complete eye closure at night is important not just to create a seal preventing tear evaporation but also to allow the ocular surface time to regenerate after extended environmental exposure during waking hours.29 Nocturnal exposure is a frequent and easily established finding in many patients.
In my experience, it occurs in as many as 40 percent of patients presenting with dry eye complaints and may be a sign of underlying tear instability as a primary cause or contributor to dry eye signs and symptoms.
Related: Explore the relationship between dry eye and sleep Exposure is discovered simply by asking the patient if he experiences dryness in the middle of the night or upon waking. It is confirmed by assessing lid closure by observation with a transilluminator or penlight with the head tilted back about 30 degrees.
A horizontal band of fluorescein staining, corresponding to the area of exposure-more commonly seen in patients who have a poor Bell’s phenomenon-is highly suggestive of nocturnal exposure.
Lagophthalmos can be quite severe. Some patients will share that they sleep with a bottle of artificial tear drops on their nightstand. It is also more severe in patients who have greater tear instability or those using forced air breathing devices that seal poorly and blow air over the exposed ocular surface.30
Sleeping with eyes partially or mostly open is a common occurrence with infants and children and is considered harmless with no damage occurring likely because of greater natural tear stability in this age group.
Management for poor nocturnal lid closure has traditionally been petrolatum-based ointments; however, they are variably effective, messy, and difficult to remove. I currently use EyeSeals 4.0 (EyeEco), which creates a tight seal and maintains high moisture levels throughout the night for most patients.
In my expereince, restoring protection at night can produce improvement in these patients with reduction of symptoms on waking and throughout the remainder of the day.
Conclusion
What eyecare practitioners calls dry eye appears to be simple, but it is complex, reflecting the critical role the tears and ocular surface play in human function. An over-simplistic view often results in clinical failure, but an over-complicated approach is equally perilous.
Understanding the underlying disease and targeting the causes of tear dysfunction rather than the subsequent signs and symptoms is a more effective strategy for managing this rapidly growing patient population.
Read more dry eye content
References:
1. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017 Jul;15(3):276-283.
2. Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA, BenÃtez-del-Castillo J, Boboridis KG, Merayo-Lloves J, Rolando M, Labetoulle M. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016 Mar;100(3):300-6.
3. Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O’Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC; Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006 Sep;25(8):900-7.
4. Korb DR, Blackie CA. “Dry Eye” Is the Wrong Diagnosis for Millions. Optom Vis Sci. 2015 Sep;92(9):e350-4
5. Hirschberg J. The History of Ophthalmology, Vol. 1: Antiquity. Translated by FC Blodi. Bonn, West Germany: Verlag JP Wayenborgh, 1982.
6. Farris RL, Stuchell RN, Nisengard R. Sjogren’s syndrome and keratoconjunctivitis sicca. Cornea. 1991 May;10(3):207-9.
7. Wolff E. The Anatomy of the Eye and Orbit, 4th ed. London: H.K. Lewis and Co.;1954:49.
8.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012 May; 31: 472-8.
9. Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1922-9.
10. Nattis A, Perry HD, Rosenberg ED, Donnenfeld ED. Influence of bacterial burden on meibomian gland dysfunction and ocular surface disease. Clin Ophthalmol. 2019 July 12;13:1225–1234.
11. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids--a review. Curr Eye Res. 2008 May;33(5):405-20.
12. Greiner JV, Glonek T, Korb DR, Booth R, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalmic Res. 1996;28(1):44-9.
13. Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, Papas EB, Rolland JP, Schmidt TA, Stahl U, Suarez T, Subbaraman LN, Uçakhan OÃ, Jones L.TFOS DEWS II Tear Film Report. Ocul Surf. 2017 Jul;15(3):366-403.
14. Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015 Dec 17;15 Suppl 1:156.
15. Thode AR, Latkany RA. Current and Emerging Therapeutic Strategies for the Treatment of Meibomian Gland Dysfunction (MGD). Drugs. 2015 Jul;75(11):1177-85.
16. Jaiswal S, Asper L, Long J, Lee A, Harrison K, Golebiowski B. Ocular and visual discomfort associated with smartphones, tablets and computers: what we do and do not know. Clin Exp Optom. 2019 Jan 21. doi: 10.1111/cxo.12851. [Epub ahead of print]
17. Sheppard AL, Wolffsohn JS. Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018 Apr 16;3(1):e000146.
18. Liu Y, Kam WR, Sullivan DA. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea. 2016 Aug;35(8):1122-6.
19. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011 May;93(5):950-62.
20. Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka-Flynn LB, Hardten DR, Lin MC, Shtein RM. n–3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med. 2018 May 3;378(18):1681-1690.
21. Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, Matossian C, Lane SS, Toyos M, Bucci FA Jr, Perry HD. Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea. 2016 Sep;35(9):1185-91.
22. Suzuki T. Inflamed Obstructive Meibomian Gland Dysfunction Causes Ocular Surface Inflammation. Invest Ophthalmol Vis Sci. 2018 Nov 1;59(14):DES94-DES101.
23. Rynerson JM, Perry HD. DEBS – a unification theory for dry eye and blepharitis.Clin Ophthalmol. 2016 Dec 9;10:2455-2467.
24. Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986 Apr;27(4):486-91.
25. Stroman DW, Mintun K, Epstein AB, Brimer CM, Patel CR, Branch JD, Najafi-Tagol K. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017 Apr 13;11:707-714.
26. Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012 Mar;34(2):237-59.
27. Epstein A, Pang L, Najafi-Tagol K, Najafi R, Stroman D, Debahov D. Comparison of Bacterial Lipase Activity in the Presence of Eye Lid Cleansers. Invest Ophthalmol Vis Sci. 2015 Jun;56(7):4446.
28. Del Rosso JQ, Bhatia N. Status Report on Topical Hypochlorous Acid: Clinical Relevance of Specific Formulations, Potential Modes of Action, and Study Outcomes. J Clin Aesthet Dermatol. 2018 Nov;11(11):36-39.
29. Katz J, Kaufman HE. Corneal exposure during sleep (nocturnal lagophthalmos). Arch Ophthalmol. 1977 Mar;95(3):449-53.
30. White DE. BLOG: CPAP and dry eye: A unique situation. Available at: https://www.healio.com/ophthalmology/cornea-external-disease/news/blogs/%7Ba2da83b9-d740-46bd-975d-c44fa9653e85%7D/darrell-e-white-md/blog-cpap-and-dry-eye-a-unique-situation. Accessed 9/16/19.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.