Novel uses of technology for systemic disease
Go beyond dilated exams and macular optical coherence tomography
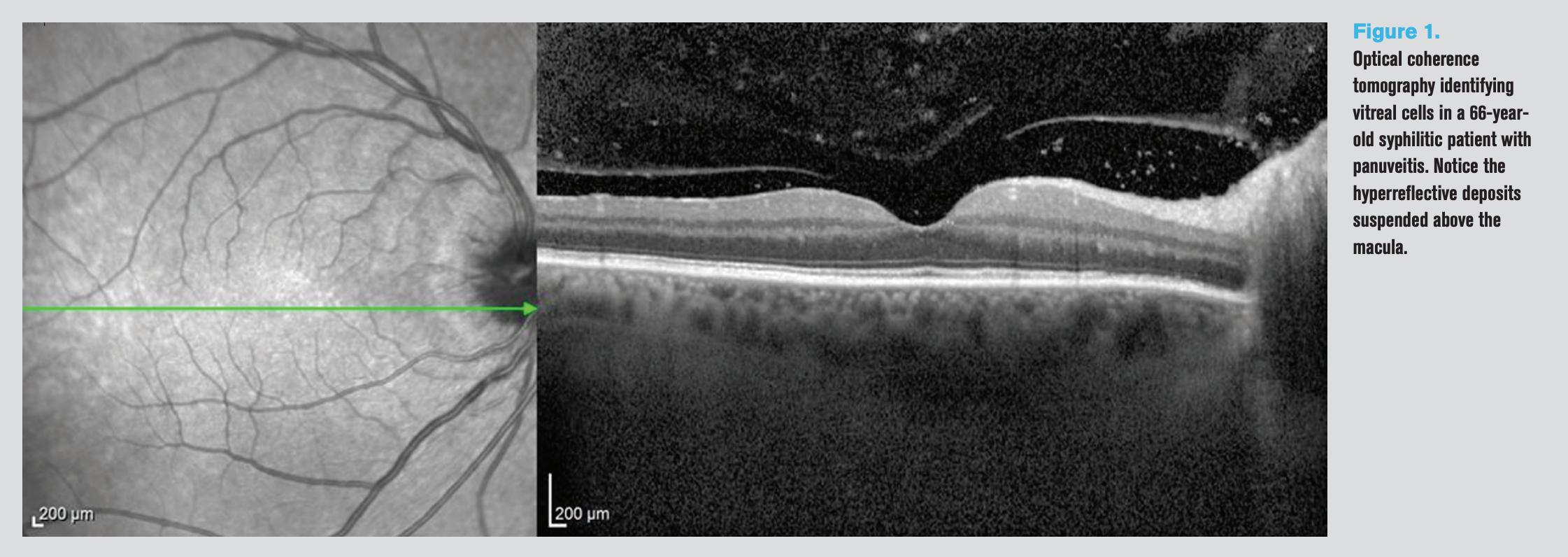
Figure 1. Optical coherence tomography identifying vitreal cells in a 66-yearold syphilitic patient with panuveitis. Notice the hyperreflective deposits suspended above the macula.

Current technology available to most optometrists, such as optical coherence tomography, specular microscopy and vitreoretinal imaging can be used for broader applications than traditional diagnostic implementations. Diagnosing and treating diseases such as Parkinson’s, Multiple Sclerosis, diabetes, and Crohn’s disease, among other systemic conditions, can be improved upon with tools that scan the the eye.
Especially today, we live in a rapidly advancing age of technology. Our computers and testing technology are becoming smarter, more multi-functional, multi-dimensional, and cross sensitive. As technology evolves, so could ODs’ creativity with its diagnostic use—to make the most of what are undoubtedly expensive office purchases and expand patient communication and illustration.
ODs are often an entry portal for patients not only into eye care but often to the healthcare system, and existing technology could be working harder for us, especially when it comes to systemic diseases.
Going beyond
ODs were taught and practice diagnostic approaches to assess retinal involvement in diabetes (DM) and hypertension (HTN) with dilated examinations and macular optical coherence tomography (OCT). But for what other conditions could we find these same imaging modalities useful?
We were taught we could visualize a posterior vitreous detachment (PVD) with a vitreous scan— but how else could we utilize these images for other systemic conditions?
We routinely perform endothelial cell counts (ECCs) on patients with Fuchs’ endothelial corneal dystroph in preparation for various ocular surgeries, but for which other diseases could it be of use?
We have so many capabilities at our literal fingertips, the possibilities are sometimes much wider and more useful than we imagine.
OCT
Findings on OCT—whether measuring macular or retinal nerve fiber layer thickness—can be an extraordinarily useful window not only for vascular diseases but also into neurological conditions.
One example is Parkinson’s disease (PD). Though it is one of the most wellknown motor symptom diseases, only recently has the spectrum of its non-motor symptoms come to light.1 In addition to a slew of ophthalmologic signs and symptoms such as double vision, dry eye, and visual hallucinations, patients with PD can also present with scannable retinal findings.2 As the retina is an extension of the central nervous system (CNS), posterior pole findings are now becoming potential useful biomarkers for neurologic changes in PD.1
Recent studies have found that these patients may exhibit thinning in the macula, specifically the forveal pit, and may prognosticate earlier diagnosis and staging.3 Specifically, it has been noted that a reduction of macular volume and foveal thickness is often found, except in the nasal and inferior inner quadrants of the macula. More often, thinning of the general retinal nerve fiber layer (RNFL), specifically in the inferotemporal quadrant, can be an indication of PD.3,4
Because PD patients often complain of reduction in visual function despite normal visual acuity, demonstrating changes via tangible numbers and visually appreciable scans could be validating and in fact quite helpful.
Multiple sclerosis (MS) is another well-known neurodegenerative disease of the CNS that has reported correlation between axonal retinal changes in the optic nerve of the retina and neurologic changes.
Not only can ODs use OCT in cases of existing diagnosis to confirm a history of previous acute optic neuritis attacks to help guide treatment algorithms, but measurements of RNFL thickness obtained with OCT could measure subclinical accumulation of axonal damage and neuronal loss.5,6 Early changes may provide an opportunity for timely intervention and introduction to broad resources prior to true clinical exhibition of the disease.6
This same hard-working retinal scanning machine often has vitreous scanning capabilities. Although ODs have been trained to examine the vitreous both with direct slit lamp and indirect biomicroscopy examination, vitreous haze can often be difficult to appreciate in the face of a compromised anterior segment and can also vary between practitioners.
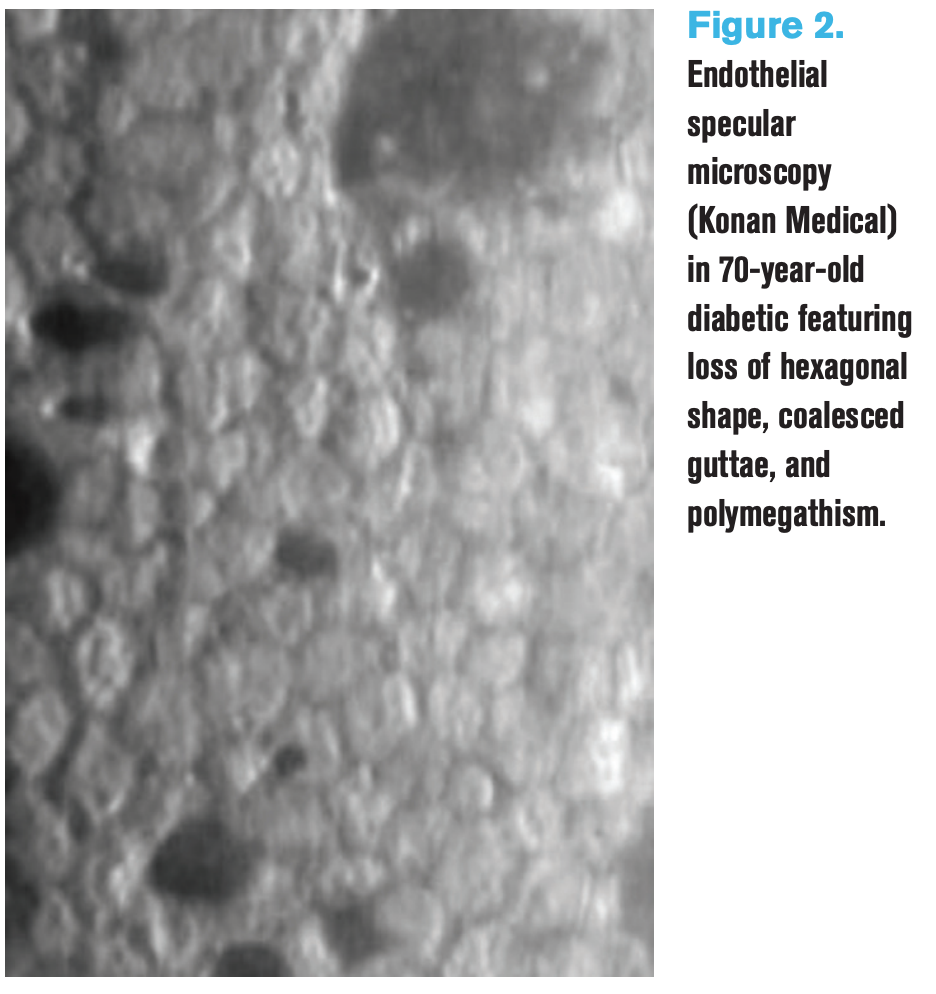
Figure 2. Endothelial specular microscopy (Konan Medical) in 70-year-old diabetic featuring loss of hexagonal shape, coalesced guttae, and polymegathism.
An OCT is able to visualize the status of the vitreous, which can be an important tool not only for classification, standardization, document prognosis and management, and for documentation and patient demonstration.7
One example in which visualization of the vitreous can play a crucial role in accurate diagnosis and management is uveitis.8 The diagnosis of anterior, intermediate, posterior uveitis depends on the anatomic location and grade of inflammation. Sarcoidosis, one of the leading causes of inflammatory eye diseases, presents with ocular involvement in 20 to 30 percent of cases.8 It was reported in one study that in eyes with sarcoid uveitis, 28 percent were anterior, 38 percent were intermediate, 12 percent were posterior, and 22 percent were panuveitis.9
In these patients, scanning the vitreous, or vitreoretinal interface, as an objective measurement would be helpful for accurate diagnosis in 72 percent of the patients, especially since posterior uveitis can sometimes be asymptomatic for the patient comparative to anterior uveitis.9 See Figure 1 for scan of vitreoretinal interface depicting vitreal inflammatory cells.
Other systemic conditions where vitreoretinal imaging can be helpful are Vogt-Koyanagi-Harada (VKH), ocular toxocarosis, and Behçet´s disease; less common but also present are acute retinal necrosis (ARN), Whipple’s disease, and Crohn’s disease.10
Moving to the anterior segment of the eye, many systemic diseases that can cause retinopathy can also affect the morphology and function of the cornea.11 There is limited information on endothelial layer function in the face of vascular pathology, although there have been suggested links.12 Examples like diabetes with its chronic hyperglycemia can also compromise the corneal endothelium, not only altering its transparency but raising for discussion its ability to heal after surgical intervention, such as cataract surgery.12
Specular microscopy
We use specular microscopy to evaluate the corneal endothelium with parameters that we are familiar with—cell density, polymegathism, and pleomorphism. Most often we use it to examine patients with Fuchs’ endotheliuam corneal dystrophy (FECD) or concurrently with glaucoma or uveitis, checking for guttae. However, we can expand its use in a helpful way to many other systemic diseases which may have corneal manifestations.
Many studies have found reduction in endothelial cell density with a higher rate of pleomorphism and polymegathism, some studies even correlating with grades of diabetic retinopathy.11,13 See Figure 2 for specular microscopy of a diabetic patient’s endothelium.
Corneal complications in diabetic patients are so common that it has been collectively termed diabetic keratopathy, which includes both epithelial and endothelial dysfunction.11
In one study, it was found that patients with either hypertension or cerebrovascular diseases developed corneal endothelial insufficiency (CEI) after cataract surgery.12 As it is known that endothelial cell counts decrease with age, it may be helpful for patients, especially with these two conditions, to illustrate the risk in a discussion that includes a visual.
Offering systemic care
ODs will always have standard clinically indicated and reimbursable reasons to complete certain scans for various diseases; however, there are potentially scores of existing ophthalmologic tests that could be useful in discussions of systemic manifestations with patients, both in a well-known and in a less well-known manner.
If ODs already have the technology in the office and are able to spend a few more minutes illustrating and discussing the results, the demonstration of full care can go a long way in systemically caring for a patient.
References
1. Sung MS, Choi S, Kim J, Ha JY, Kim BC, Heo H, Park SW. Inner retinal thinning as a biomarker for cognitive impairment in de novo Parkinson’s disease. Sci Rep. 2019 Aug 14;9(1):11832.
2. Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ. Ophthalmologic features of Parkinson’s disease. Neurology. 2004 Jan 27;62(2):177-80.
3. Simao LM. The contribution of optical coherence tomography in neurodegenerative diseases. Curr Opin Ophthalmol. 2013 Nov;24(6):521-7.
4. Aydin TA, Umit D, Nur OM, Fatih U, Asena K, Nefise OY, Serpil Y. Optical coherence tomography findings in Parkinson’s disease. Kaohsiung J Med Sci. 2018 Mar;34(3):166-171.
5. Pérez Del Palomar A, Cegoñino J, Montolío A, Orduna E, Vilades E, Sebastián B, Pablo LE, Garcia-Martin E. Swept source optical coherence tomography to early detect multiple sclerosis disease. The use of machine learning techniques. PLoS One. 2019 May 6;14(5):e0216410.
6. Costello F, Burton JM. Retinal imaging with optical coherence tomography: a biomarker in multiple sclerosis? Eye Brain. 2018 Jul 31;10:47-63.
7. Keane PA, Karampelas M, Sim DA, Sadda SR, Tufail A, Sen HN, Nussenblatt RB, Dick AD, Lee RW, Murray PI, Pavesio CE, Denniston AK. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology. 2014 Sep;121(9):1706-14.
8. Onal S, Tugal-Tutkun I, Neri P, Herbort CP. Optical coherence tomography imaging in uveitis. Int Ophthalmol. 2014 Apr;34(2):401-35.
9. Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996 Nov;103(11):1846-53.
10. Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57.
11. Shenoy R, Khandekar R, Bialasiewicz AA, Muniri AA. Corneal Endothelium in Patients with Diabetes Mellitus: A Historical Cohort Study. Eur J Ophthalmol. May-Jun 2009;19(3):369-75.
12. Guvenmez O, Kayiklik A. Hypertension and Cerebrovascular Diseases Are Related to Corneal Endothelial Insufficiency—a Retrospective Study. Ophthalmology J. 2019;4:11-14.
13. Pardos GJ, Krachmer JH. Comparison of endothelial cell density in diabetics and a control population. Am J Ophthalmol. 1980 Aug;90(2):172-4.

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.