OCT biomarkers associated with advanced age-related macular degeneration
Imaging can elucidate visual prognosis and disease progression risk.
Age-related macular degeneration (AMD) is a multifactorial, progressive retinal disease and a leading cause of visual impairment. About 200 million people worldwide have AMD, and this number is expected to increase by nearly 50% over the next 20 years.1 It is critical that eye care providers accurately diagnose, educate, and monitor their AMD patients. Optical coherence tomography (OCT) is a useful tool to assess and track macular changes due to AMD, and OCT biomarkers can provide clues to help inform visual prognosis and progression risk.
OCT biomarkers are structural changes that can result from fluid (exudative AMD) or from alterations in the retinal layers (exudative or nonexudative AMD).2 Numerous biomarkers have been identified for advanced AMD. These biomarkers can aid in staging AMD, making treatment decisions, and assessing the likelihood of future progression.
Figure 1. Double layer sign. Figure 2. Subretinal drusenoid deposit marked by blue arrow; hyperreflective foci highlighted by blue circle. Figure 3. Hyporeflective wedge. Figure 4. Outer retinal tubulation. (Images courtesy of Srinivas Kondapalli, MD.)
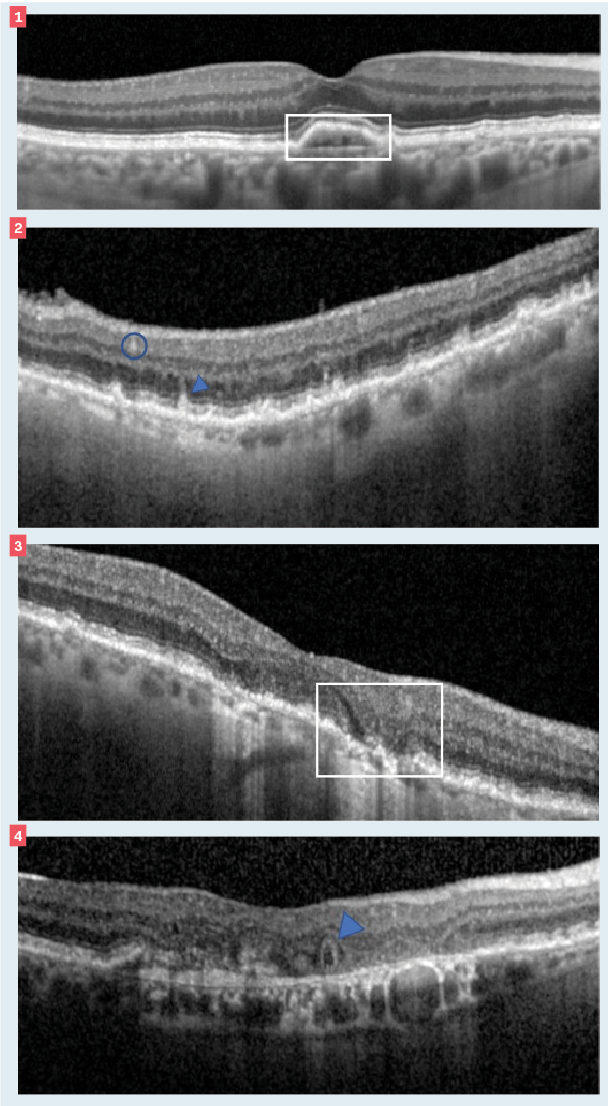
Two biomarkers for exudative AMD (eAMD) are double layer sign (DLS) and fellow eye choroidal neovascularization (CNV). DLS can be seen on OCT as a separation between the retinal pigment epithelium (RPE) and Bruch’s membrane (BM) due to a shallow irregular RPE detachment (SIRE). Both RPE and BM are reflective on OCT, and their separation creates the appearance of a double layer.3 The presence of a DLS is associated with the potential for visual distortion and an increased risk (15-fold) of exudation4; thus, patients with a DLS should be monitored closely.
CNV in 1 eye correlates to an increased risk for CNV development in the fellow eye. One large study found an overall conversion rate of about 10% per year.5 The contralateral eye of any patient with active CNV or a history of it should be routinely checked for the development of exudation. Additionally, these patients may benefit from AREDS 2 supplements, which have been shown to reduce the risk of developing CNV.6
Two biomarkers for nonexudative AMD are subretinal drusenoid deposits (SDDs) and hyporeflective wedges. SDDs, also called reticular pseudodrusen, are mound- or conical-like projections between the RPE and photoreceptors (remember, classical drusen are below the RPE). Patients with SDDs may have reduced dark adaptationand are at an increased risk for the development of geographic atrophy (GA).7,8
Abnormalities in the inner and outer retinal layers can lead to several signs on the OCT, including a hyporeflective wedge. These appear as a gull-wing shape and denote atrophy of the outer layers. Hyporeflective wedges are highly correlated with a risk to GA progression.7
Two biomarkers with prognostic value for both eAMD and GA are outer retinal tubulations (ORT) and hyperreflective foci. ORTs are round/ovoid areas of hyporeflectivity surrounded by a hyperreflective border.9
ORTs are associated with a worse visual prognosis in patients with eAMD10 as well as slowing of the growth rate of advanced subfoveal GA lesions.11
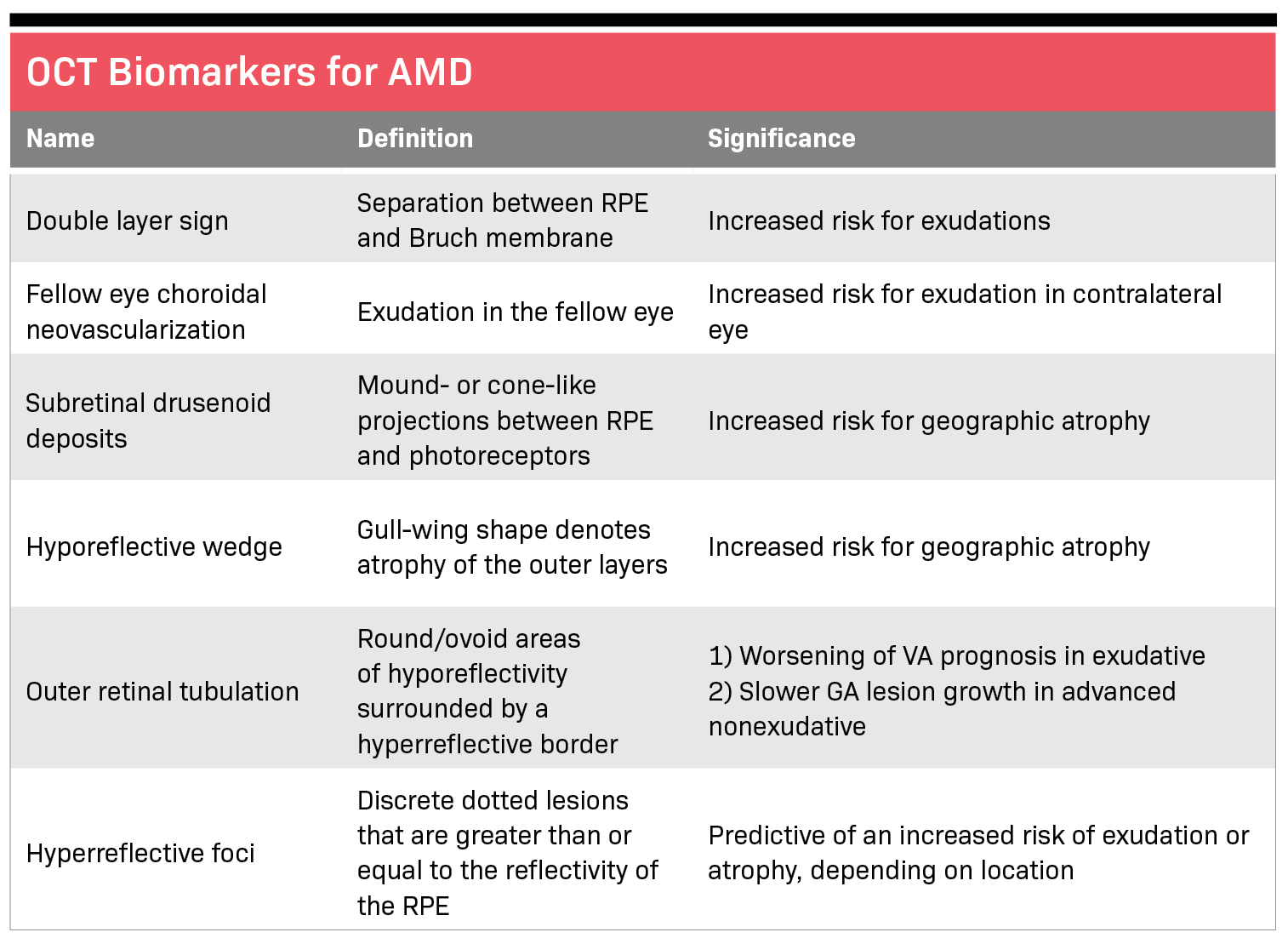
Hyperreflective foci (HRF) are discrete dotted lesions that are greater than or equal to the reflectivity of the RPE. They can appear singly or grouped.8 HRF may be predictive of an increased risk of exudation or atrophy depending on its location12—the greater the number and distribution of HRF, the greater the risk of progression.8

Identifying these OCT biomarkers can provide prognostic information to better inform clinical decision-making, patient education, and referrals. As the ever-evolving AMD landscape continues to offer a better understanding of pathogenesis, prevention, and new therapeutic options, optometrists will play an important role in recognizing patients that may benefit from these advances.
References
1. Deng Y, Qiao L, Du M, et al. Age-related macular degeneration: epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021;9(1):62-79. doi:10.1016/j.gendis.2021.02.009
2. Metrangolo C, Donati S, Mazzola M, et al. OCT biomarkers in neovascular age-related macular degeneration: a narrative review.
J Ophthalmol. 2021:9994098. doi:10.1155/2021/9994098
3. Shi Y, Motulsky EH, Goldhardt R, et al. Predictive value of the OCT double-layer sign for identifying subclinical neovascularization in age-related macular degeneration. Ophthalmol Retina. 2019;3(3):211-219. doi:10.1016/j.oret.2018.10.012
4. de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-266. doi:10.1016/j.ophtha.2017.08.030
5. Maguire MG, Daniel E, Shah AR, et al. Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(10):2035-2041. doi:10.1016/j.ophtha.2013.03.017
6. Chew EY, Clemons TE, Agrón E, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 2014;132(3):272-277. doi:10.1001/jamaophthalmol.2013.6636
7. Monge M, Araya A, Wu L. Subretinal drusenoid deposits: an update. Taiwan J Ophthalmol. 2022;12(2):138-146. doi:10.4103/tjo.tjo_18_22
8. Jaffe GJ, Chakravarthy U, Freund KB, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: classification of atrophy meeting report 5. Ophthalmol Retina. 2021;5(9):855-867. doi:10.1016/j.oret.2020.12.009
9. Arrigo A, Aragona E, Battaglia O, et al. Outer retinal tubulation formation and clinical course of advanced age-related macular degeneration. Sci Rep. 2021;11:14735. doi:10.1038/s41598-021-94310-5
10. Lee JY, Folgar FA, Maguire MG, et al. Outer retinal tubulation in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2014;121(12):2423-2431. doi:10.1016/j.ophtha.2014.06.013
11. Hariri A, Nittala MG, Sadda SR. Outer retinal tubulation as a predictor of the enlargement amount of geographic atrophy in age-related macular degeneration. Ophthalmology. 2015;122(2):407-413. doi:10.1016/j.ophtha.2014.08.035
12. Fragiotta S, Abdolrahimzadeh S, Dolz-Marco R, Sakurada Y, Gal-Or O, Scuderi G. Significance of hyperreflective foci as an optical coherence tomography biomarker in retinal diseases: characterization and clinical implications. J Ophthalmol. 2021:6096017. doi:10.1155/2021/6096017

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.