Phakic IOL implantation: Visual and refractive outcomes
Posterior chamber pIOLs should be considered for patients in which other refractive surgeries may not be an option and as an reversible alternative with fewer complications.
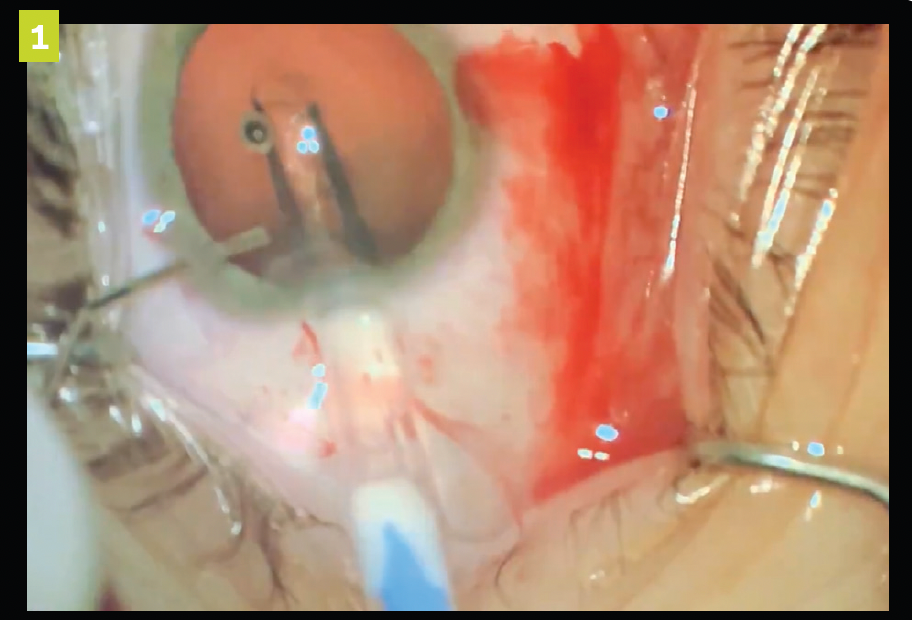
Figure 1. Implantable contact lens (ICL) injection into the anterior chamber through clear corneal incision.
When optometrists think of refractive surgery options for patients, the immediate choices that come to mind are laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK).
These come with patient-selection limitations and increased risk of dry eye, night vision disturbances, and possible scarring.
Another underutilized, well-performing option is implantable collamer lens, or implantable contact lens (ICL), which is a posterior chamber phakic intraocular lens (pIOL).

Although the idea of this procedure has been around since the 1950s, it has not been something we consider mainstream treatment for elective refractive surgery.
Related: Comanaging intraocular lens power calculations
An increasing number of surgeons are performing pIOL implants because they do not damage or change the cornea. It is important to know how to comanage pIOLs to achieve successful treatment outcomes.
History of pIOLs
Developed in 1953, the first implanted anterior chamber pIOL was a minus-powered lens that was supported by the angle-correcting myopia.1
This first iteration came with many complications, including endothelial damage, pupillary irregularities, and angle fibrosis.1
Another attempt was made in the 1980s, by Merab Dvali, MD, PhD, and Georges Baikoff, MD, utilizing a similar angle-supporting principle.1
Improvements in both the material and haptic design allowed it to be more flexible and thinner, and have less complications, but it still led to pupillary and angle changes.
A different treatment emerged in 1986 by Svyatoslav Fyodorov, MD: the first posterior chamber pIOL. The original design had the optic in the anterior chamber with the haptics behind the iris, followed by a rectangular silicone plate IOL.
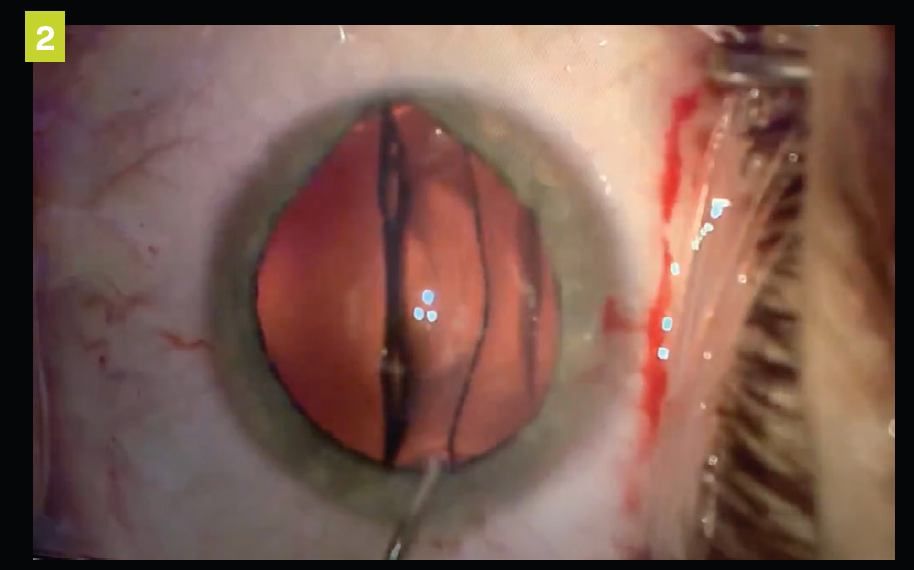
Figure 2. ICL placement between the natural crystalline and the iris.
Complications, including inflammation and cataracts, led to the discontinuation of these lenses.1
Next came the Phakic Refractive Lens (CIBA Vision), which was a 1-piece hydrophobic silicone elastomer that floated above the crystalline lens, and the haptics rested on the zonules. Unfortunately, this attempt resulted in zonular dehiscence and subluxation of the lens.
Fast forward to 1993, and the Visian ICL (STAAR Surgical Co) was introduced, which is currently the only posterior chamber pIOL that is approved by the US Food and Drug Administration (FDA) for use in the United States.
Related: A guide to the latest presbyopia-correcting IOLs
The current ICL, version 4, was released in 1999 and is made from a copolymer of hydroxyethyl methacrylate and porcine collagen, called a collamer.1 It is placed behind the iris and in front of the crystalline lens within the posterior chamber of the eye.
The ICL doesn’t rest on the natural lens but sits in front of an area between the anterior portion of the crystalline lens and the posterior surface of the ICL, known as the vault.2 It comes in a multitude of diameter sizes to achieve this vault and accounts for human variance.2
Range of treatment for myopia is full correction from —3 to —15 diopter (D) and reduction of myopia from —15 to —20 D, with less than or equal to 2.5 D astigmatism.1,3
The Visian Toric ICL is currently in trials for FDA approval, with a proposed range of 1 to 4 D of astigmatism and —3 to —20 D of myopia.1,2
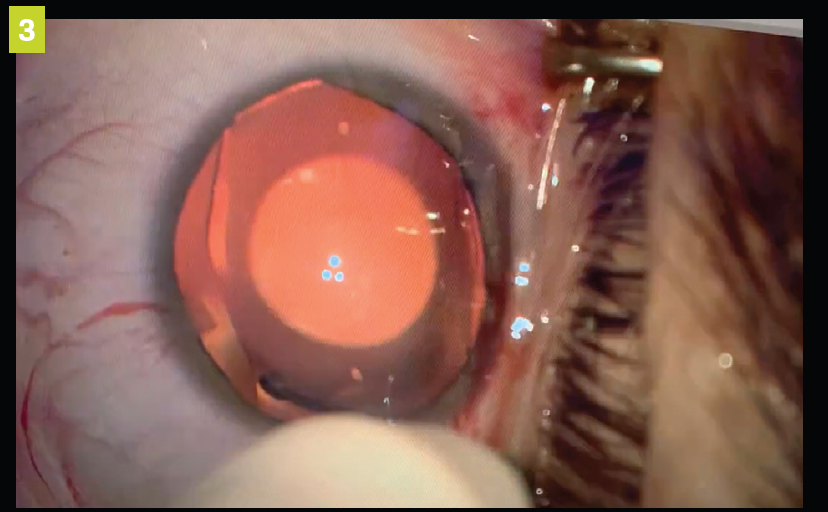
Figure 3. ICL beginning to open once placed in the anterior chamber.
Evaluation
With refractive surgery, it is important to properly evaluate the patients to decide who is an appropriate candidate to ensure safety and positive outcomes.
One could argue that the evaluation for ICL is even more comprehensive than that of other refractive surgeries.
Good candidates should have refractive stability, less than 0.5 D change within the past 6 to 12 months, open angles assessed by gonioscopy or optical coherence topography, as well as healthy endothelium.1
Related: How OSD affects advancted technology IOLs
Patients who would not be good candidates are those who have narrow angles, pigment dispersion syndrome, or pseudoexfoliation syndrome; are already at risk for glaucoma; or have any posterior ocular disease.1,3
When referring for an evaluation, make sure the patient discontinues soft contact lens wear 1 week prior to the appointment and toric soft lens or rigid gas permeable lens wear at least 2 weeks prior.1
Preoperative examination by the surgeon will include dilated refraction, central corneal thickness measurement, endothelial cell count, keratometry, axial eye lengths, tonometry, and mesopic pupil diameter.
Special attention is paid to the anterior chamber depth because it is critical to the safety of the posterior chamber pIOL implantation.
Minimum anterior chamber depth between the endothelium and the central anterior lens capsule should measure 3.0 to 3.2 mm.1
The determination of the size of the ICL implant is based on the desired vault. This is very important to avoid possible complications.
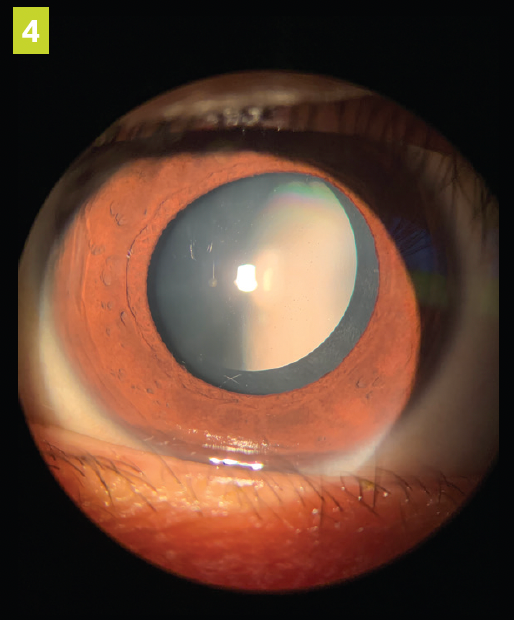
Figure 4. ICL at dilated post operative examination after 1 month. (All images courtesy of Cecelia Koetting, OD, FAAO, Diplomat ABO)
An incorrect vault can lead to pupillary block, anterior subcapsular cataracts (ASC), pigment dispersion syndrome, or glaucoma.
Studies have shown an increased risk for ASC if the vault is less than 0.9 mm, along with higher levels of myopia.3 These studies have led to the conclusion that 0.15 mm should be considered the lower limit of a safe central vault.3
Surgery and aftercare
Prior to the implantation of the ICL, the patient has either laser or operative peripheral iridotomies to help prevent pupillary block.1 The pupil is dilated prior to surgery and is performed similarly to cataract surgery under topical anesthesia.
Related: How the tear film affects IOL measurements
After the anterior chamber is filled with an ophthalmic viscosurgical device (OVD), the pIOL is injected into the anterior chamber through a clear corneal incision. The ICL is then allowed to unfold, and footplates are gently tucked beneath the iris (see Figures 1-4).
Some surgeons keep the patient in the surgery center for an IOP check at 1 hour post procedure before allowing them to check out. If the IOP is elevated, oral acetazolamide may be given.
After surgery, the patients are typically monitored closely at 1-day, 1-week, and 1-month intervals to ensure there is no angle closure or iris bombe, resulting in elevated IOP.1,3
Both surgeons I have comanaged with on ICL patients have preemptively put the patients on brimonidine 0.1% twice daily in the operative eye for 1 week.
This is the most concerning possible adverse effect within the first few days of surgery. To mitigate other possible complications, patients are placed on topical corticosteroids and antibiotics.
The benefits
Visual outcomes with the ICL typically show improvement. Studies show the postoperative uncorrected visual acuity (UCVA) is 20/20 or better in 97% at 1 month and 100% at 3, 6, 12, and 36 months.3
Compared with rates of other refractive surgeries, complication rates were lower and visual outcomes were overall better with the ICL groups.
Three retrospective studies comparing LASIK and ICL noted better visual outcomes in patients with high myopia treated with ICL.4-6
They also noted a higher measurement of safety, predictability, and stability.4-6 This reinforces ICL as a viable alternative for patients with either low or high myopia.
Prospective studies comparing PRK and ICL in moderate to high myopic astigmats also found better performance in the ICL arm.7,8 Improved contrast sensitivity, night-driving simulation, and UCVA put ICL ahead in 2 separate studies.7,8
Related: Extended-depth-of-focus IOLs may provide improved visual visual performance
In a head-to-head study with the small incision lenticule extraction (SMILE) surgical procedure, the ICL group had significantly fewer higher order aberrations and better-quality vision (decreased halos and starbursts).9 The ICL patients also noted better refractive outcomes and percentage of eyes within 0.50 D of plano.9
When thinking long term about our patient’s visual journey, the less we change the corneal surface, the better.
It can not only lead to worsening ocular surface disease, but it can also make cataract surgery outcomes less predictable and put patients at risk for chronic corneal degeneration.
Pipeline and conclusion
EVO Visian ICL, a newer version of the Visian ICL used in Europe, is designed to help treat myopia and astigmatism. It also has strategically placed holes in the implant to reduce the risk of increased IOP and glaucoma.2
The EVO Visian ICL was also recently approved by the FDA in the US earlier this year.
It is designed to be used in phakic eye treatment for patients ages 21 to 45 for the correction/reduction of myopia in patients with spherical equivalent ranging from -3.0 D to -20.0 D at the spectacle plane, as well as the correction/reduction of myopic astigmatism in patients with spherical equivalent ranging from -3.0 D to -20.0 D with cylinder of 1.0 D to 4.0 D at the spectacle plane.
Although limitations exist with posterior chamber pIOL, remember it as an option for those patients in which other refractive surgeries may not be an option and as a reversible alternative with fewer complications.
References
1. Huang D, Schallhorn SC, Sugar A, et al. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(11):2244-2258. doi:10.1016/j.ophtha.2009.08.018
2. STAAR Visian Toric ICL post-approval study (TICL-PAS). ClinicalTrials.gov. Updated August 20, 2020. Accessed 4/5/21. https://clinicaltrials.gov/ct2/show/NCT04516772
3. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clinical Ophthalmol. 2016;10:1059-1077. doi:10.2147/OPTH.S111620
4. Kamiya K, Shimizu K, Igarashi A, Komatsu M. Comparison of Collamer toric implantable [corrected] contact lens implantation and wavefront-guided laser in situ keratomileusis for high myopic astigmatism. J Cataract Refract Surg. 2008;34(10):1687-1693.
5. Sanders D, Vukich JA. Comparison of implantable collamer lens (ICL) and laser-assisted in situ keratomileusis (LASIK) for low myopia. Cornea. 2006;25(10):1139-1146. doi:10.1097/ICO.0b013e31802cbf3c
6. Sanders DR. Matched population comparison of the Visian Implantable Collamer Lens and standard LASIK for myopia of -3.00 to -7.88 diopters. J Refract Surg. 2007;23(6):537. doi:10.3928/1081-597X-20070601-02
7. Schallhorn S, Tanzer D, Sanders DR, Sanders M, Brown M, Kaupp SE. Night driving simulation in a randomized prospective comparison of Visian toric implantable collamer lens and conventional PRK for moderate to high myopic astigmatism. J Refract Surg. 2010;26(5):321-326. doi:10.3928/1081507X-20090617-09
8. Schallhorn S, Tanzer D, Sanders DR, Sanders ML. Randomized prospective comparison of visian toric implantable collamer lens and conventional photorefractive keratectomy for moderate to high myopic astigmatism. J Refract Surg. 2007;23(9):853-867. doi:10.3928/1081-597X-20071101-01
9. Siedlecki J, Schmelter V, Mayer WJ, et al. SMILE versus implantable collamer lens implantation for high myopia: a matched comparative study. J Refract Surg. 2020;36(3):150-159. doi:10.3928/1081597X-20200210-02

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.