Dry eye management may benefit patients with keratoconus
Reducing inflammation and managing corneal microtrauma are new strategies

Keratoconus is described as a bilateral but asymmetric, noninflammatory, progressive degeneration of the cornea, leading to extreme refractive errors with impaired visual acuity and conical corneal protrusion. As diagnostic approaches developed, the prevalence of the disease has increased from 1 in 2000 to 1 in 84.1 Modern diagnostic techniques include Scheimpflug imaging, corneal wavefront, pachymetry analysis, and epithelial mapping.
As diagnostic technology improves, management strategies are evolving as well. Keratoconus has historically been described as a noninflammatory degeneration. While historically, management was limited to gas permeable contact lenses, or penetrating keratoplasty in severe cases, new treatments include early corneal collagen cross-linking (CXL), scleral contact lenses, Bowman’s membrane transplants, and topography-guided photokeratoplasty with CXL. Medical management includes managing corneal microtrauma and inflammation.
Eye rubbing
Correlation of eye rubbing and keratoconus progression is commonly reported in the literature,2,3 but uncertainty exists whether the atopy leading to the eye rubbing is the etiology or if physical trauma from eye rubbing is the cause. Moran reported eye rubbing— described as beneath or inside the eye and rubbing with the knuckles, base of thumbs, or fingertips—increases risk of keratoconus. Further, keratoconus was more significantly associated with knuckle rubbing.2
Najmi reported mechanical eye rubbing causes the thinning of keratocyte. Also reported was that the corneal effect of eye rubbing depends on the period and force of eye rubbing.3
Eye rubbing may affect the cornea at a molecular level. Shetty reported eye rubbing increased the level of tear matrix metalloproteinase (MMP)-13, IL-6, and TNF-α in eyes of patients with keratoconus as well as healthy patients.4 Lema reported patients with keratoconus had significantly higher levels of inflammatory markers, including IL-6, TNF-α, and MMP-9. In addition, investigators reported the levels of these inflammatory markers increased with the severity of keratoconus.5
Contact lens microtrauma
Contact lens wear and eye rubbing increase incidence of mechanical trauma to the cornea. Addressing low levels of ocular inflammation will also improve contact lens wearing comfort and reduce lens complications. Erie reported altered keratocyte morphology and density within the anterior stroma in contact lens wearers with keratoconus.6 Lema reported contact lens wear demonstrated upregulation of IL-6, TNF- α, ICAM-1, and VCAM-1 in the tears of subjects with keratoconus, especially in gas permeable lens wearers.7
The authors suggested a progressive cycle of proinflammatory cytokines, proteolytic enzymes, and inhibitors may be responsible for microenvironmental changes found in keratoconus. The resulting imbalance may initiate and perpetuate corneal inflammatory pathways to result in the structural abnormalities seen with disease progression.7
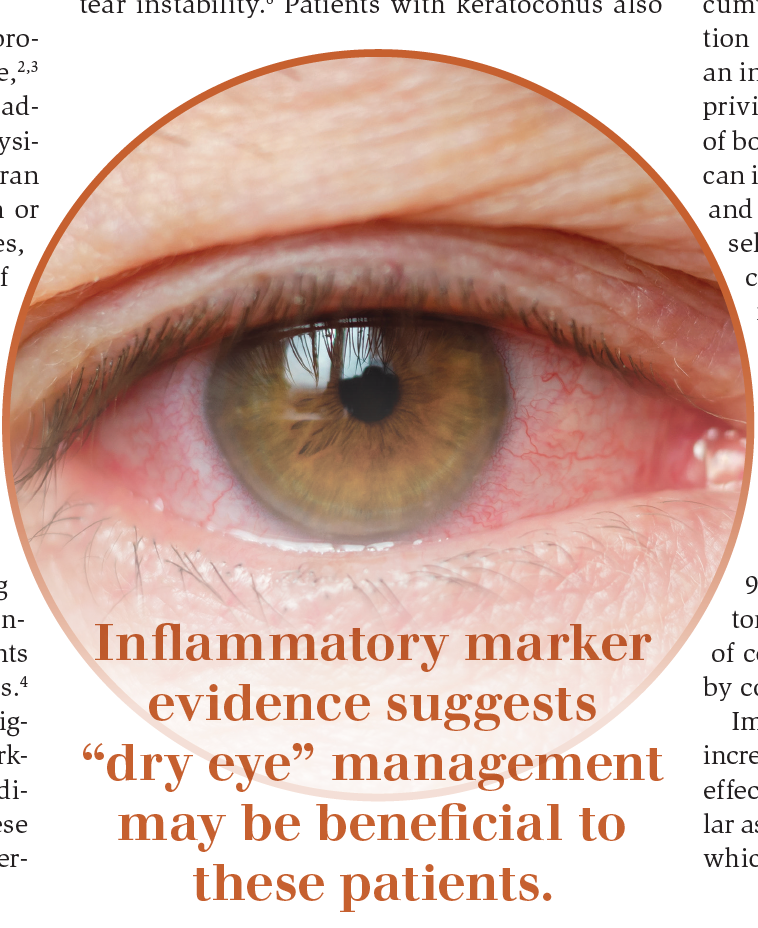
Inflammatory marker evidence suggests “dry eye” management may be beneficial to these patients. Patients with keratoconus reportedly experience greater symptoms of dry eye and greater tear instability.8 Patients with keratoconus also demonstrated significantly decreased tear secretion and higher Ocular Surface Disease Index survey scores compared with controls.9
Patients with any stage of keratoconus, including those with corneal grafts, require clinical differentiation between allergic conjunctivitis and dry eye.10 Any comorbidity that is inflammatory in nature may add synergistically to other forms of keratoconus-related inflammation and exacerbate its pathogenetic processes.11
Immune privilege
Inflamed grafts commonly reject and will do so quickly. Immunological privilege is relative and cumulative. Keratitis years prior to transplantation increases rejection risk because changes in an inflamed cornea contribute to the reduction of privilege. Human corneas carry a limited amount of bone marrow-derived cells. These special cells can initiate an immune response. The cells mature and are recruited into the cornea via limbal vessels with inflammation. Problematically, these cells persist after the acute insult resolves. The more bone marrow-derived cells present in the host cornea, the higher the graft rejection rate. The more inflammation, the less privilege.12
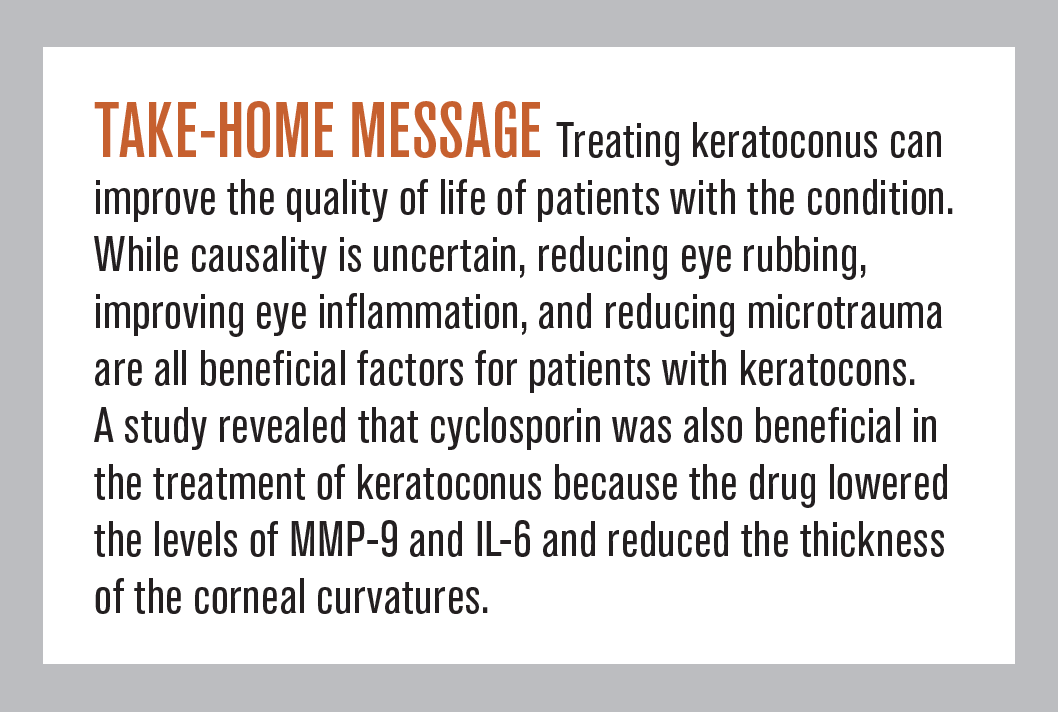
Cyclosporin was found to be beneficial in eyes with keratoconus in a study by Shetty. After topical cyclosporin was used for 6 months, an improvement in levels of MMP- 9, IL-6, and TNF-α was observed. Investigators also noted local flattening and reduction of corneal curvatures after treatment measured by corneal topography.4
Immunomodulators reduce inflammation, and increasing and stabilizing the tear film reduces the effect of irregular astigmatism. Reducting irregular astigmatism improves higher order aberrations, which may subjectively improve visual acuity.
Conclusion
Despite the historical description of keratoconus as a noninflammatory disease, inflammation may play a role in the progression of keratoconus. These data suggest that an inflammatory cycle much like that of dry eye may play an important role in keratoconus pathogenesis.
While causality may be uncertain, managing eye rubbing, reducing inflammation, and reducing corneal microtrauma may improve the quality of life for patients with keratoconus. In addition to monitoring keratoconus progression using corneal topography, refraction, and best-corrected visual acuity, use of a dry eye screening tool and measurement of inflammatory markers may be beneficial. Daily use of immunomodulators and topical steroids for symptom flares should be considered as well.
References
1. Chan E, Chong EW, Lingham G, et al. Prevalence of keratoconus based on Scheimpflug imaging: the Raine study. Ophthalmology. 2021;128(4):515-521. doi:10.1016/j.ophtha.2020.08.020
2. Moran S, Gomez L, Zuber K, Gatinel D. A case-control study of keratoconus risk factors. Cornea. 2020;39(6):697-701. doi:10.1097/ICO.0000000000002283
3. Najmi H, Mobarki Y, Mania K, et al. The correlation between keratoconus and eye rubbing: a review. Int J Ophthalmol. 2019;12(11):1775-1781. doi:10.18240/ ijo.2019.11.17
4. Shetty R, Ghosh A, Lim RR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56(2):738-750. doi:10.1167/iovs.14-14831
5. Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112(4):654-659. doi:10.1016/j. ophtha.2004.11.050
6. Erie JC, Patel SV, McLaren JW, Nau CB, Hodge DO, Bourne WM. Keratocyte density in keratoconus. A confocal microscopy study (a). Am J Ophthalmol. 2002;134(5):689-95. doi:10.1016/s0002- 9394(02)01698-7
7. Lema I, Durán JA, Ruiz C, Díez-Feijoo E, Acera A, Merayo J. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008;27(7):758-763. doi:10.1097/ICO.0b013e31816a3591
8. Carracedo G, Recchionoi A, Alejandre- Alba N, et al. Signs and symptoms of dry eye in keratoconus patients: a pilot study. Curr Eye Res. 2015;40(11):1088-1094. doi: 10.3109/02713683.2014.987871
9. Dienes L, Kiss HJ, Perényi K, et al. Corneal sensitivity and dry eye symptoms in patients with keratoconus. PLoS One. 2015;10(10):e0141621. doi:10.1371/ journal.pone.0141621
10. Sundarraj CV, Foulks GN. Managing symptoms of allergy and dry eye in patients with keratoconus. Invest Ophthalmol Vis Sci. 2005;46(13):941.
11. McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015;92(2):e35-41. doi:10.1097/ OPX.0000000000000455
12. Coster DJ, Jessup CF, Williams KA. Mechanisms of corneal allograft rejection and regional immunosuppression. Eye (Lond). 2009;23(10):1894-1897. doi:10.1038/eye.2009.17

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.