How diabetes and glaucoma intersect
Consider the impact of diabetes on management of patients with glaucoma


The estimated global prevalence of diabetes mellitus was 463 million in 2019, representing 9.3% of the world’s population with the numbers predicted to increase to 578 million by 2030.1 Diabetes is a major cause of blindness, largely because of its effect on the retina. However, diabetes also affects virtually every other structure in the eye and is a risk factor for numerous ocular pathologies. Yet its relationship to primary open-angle glaucoma is debatable despite multiple disease mechanisms that are common to both diabetes and glaucoma.2
Glaucoma is the leading cause of irreversible blindness in the world, with the number of people with glaucoma estimated to be 76.0 million in 2020 and 111.8 million in 2040.3 Results from numerous studies have demonstrated an increased risk of developing glaucoma in patients with diabetes.4-6 However, many studies have determined that having diabetes does not impact the development of glaucoma.7-9
Diabetes as a neurodegenerative disease
Diabetes has long been considered both a vasculopathic and neuropathic disease with the prevailing thinking that retinopathy is the primary event and neurodegeneration occurs subsequent to microvascular impairment. However, results from recent studies show that degeneration of the inner retina, termed diabetic retinal neurodegeneration (DRN), may precede the funduscopic signs of diabetic retinopathy.10-12
These neuropathic changes include apoptosis of retinal neuronal cells, glial cell activation, glutamate excitotoxicity, decrease in neuroprotective factors, and impairment of neurovascular coupling.2,10 DRN can be detected clinically using optical coherence tomography (OCT) to show thinning of the peripapillary retinal nerve fiber (pRNFL) layer and ganglion cell complex.10-13 These neurodegenerative changes, in addition to other impairments related to diabetes, can confound the assessment for glaucoma.
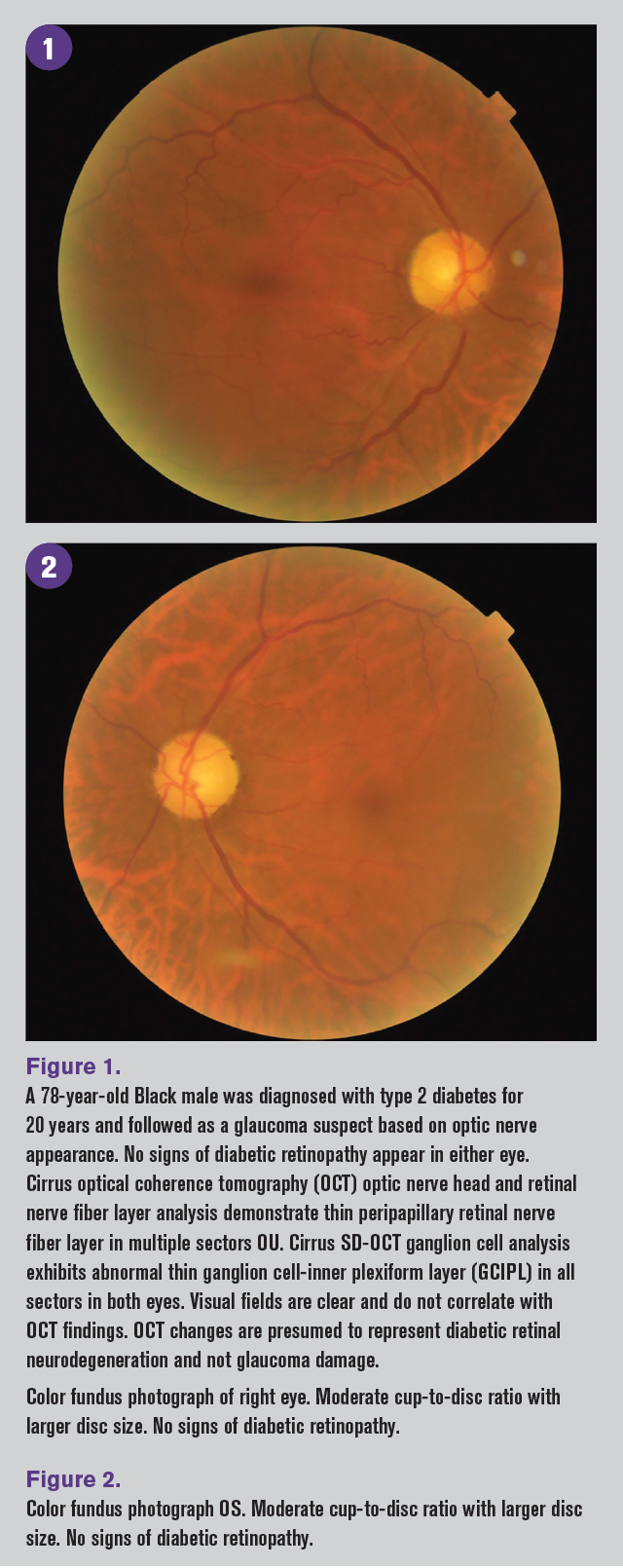
Clinical glaucoma findings
Diabetes affects both the inner and outer retinal structures with subsequent impairment, as well as other ocular structures that can potentially affect the evaluation for glaucoma. These changes can be seen clinically in intraocular pressure (IOP) measurements, pachymetry, visual field testing, and OCT scans.
IOP has been shown to be higher in patients with diabetes.5,14,15 Several hypotheses offered in support of this finding are that higher IOPs seen in patients with diabetes may be related to a slightly thicker central corneal thickness (CCT), changes in the biomechanical properties of the cornea, or a decrease in aqueous outflow because of alterations of the trabecular meshwork caused by an accumulation of advanced glycation end products.15 However, the IOP difference seen in patients with diabetes vs patients without diabetes is relatively small and likely not clinically relevant. The CCT of individuals with diabetes are also slightly thicker than those without diabetes, albeit also by a small and likely nonclinically relevant amount.15 However, results from a small cross-sectional study found the increased CCT seen with increasing severity of peripheral neuropathy to be clinically significant.16
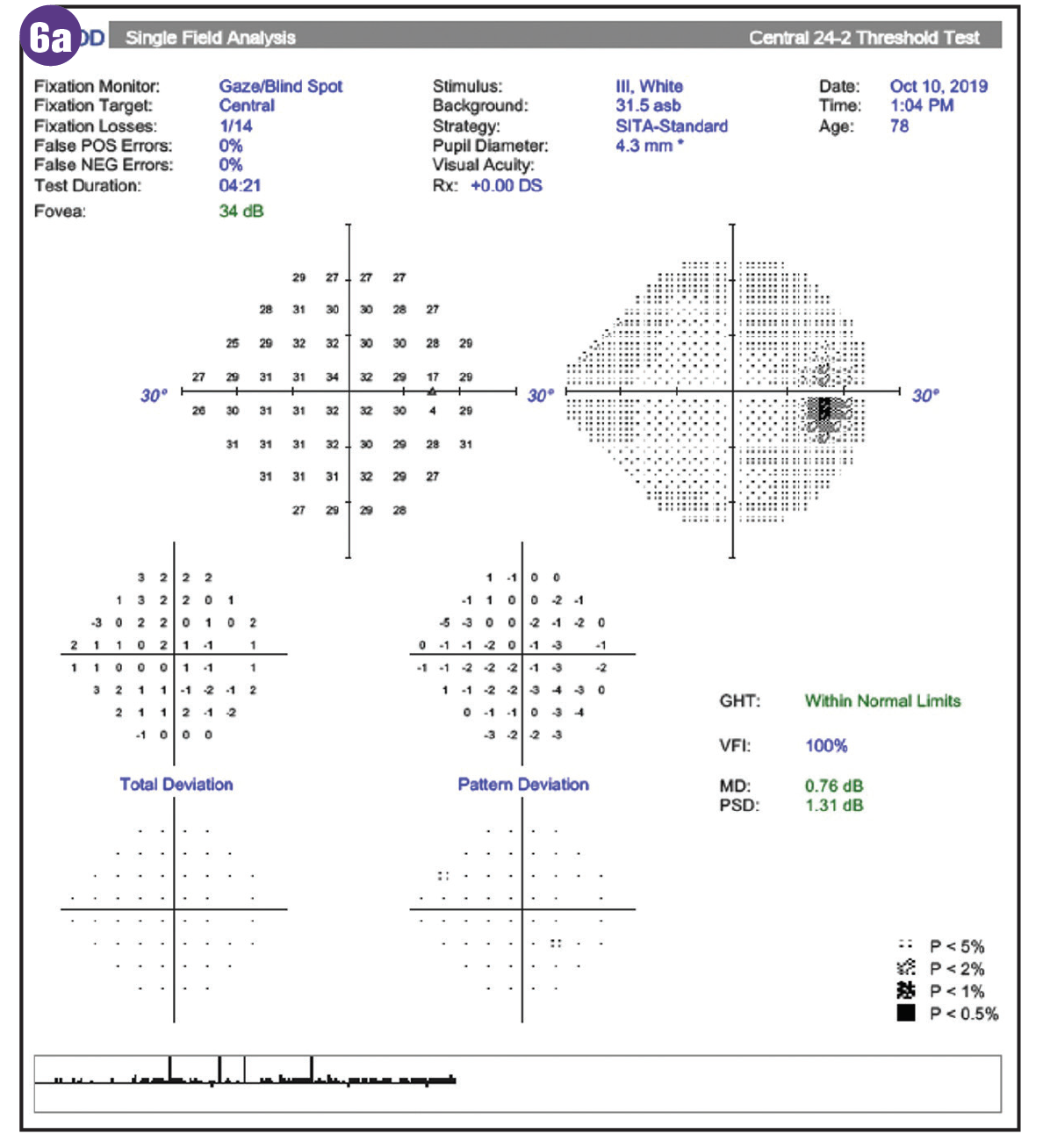
Diabetic retinopathy is often an exclusion factor in glaucoma trials largely because of the known impact of retinopathy on visual field studies and the retinal nerve fiber layer. However, visual field defects in patients with diabetes have also been shown to occur in the absence of retinopathy, suggesting that neuroretinopathy occurs prior to visible retinal lesions.18 These defects are more readily seen with frequency doubling technology and short wavelength automated perimetry vs standard automated perimetry.17-19
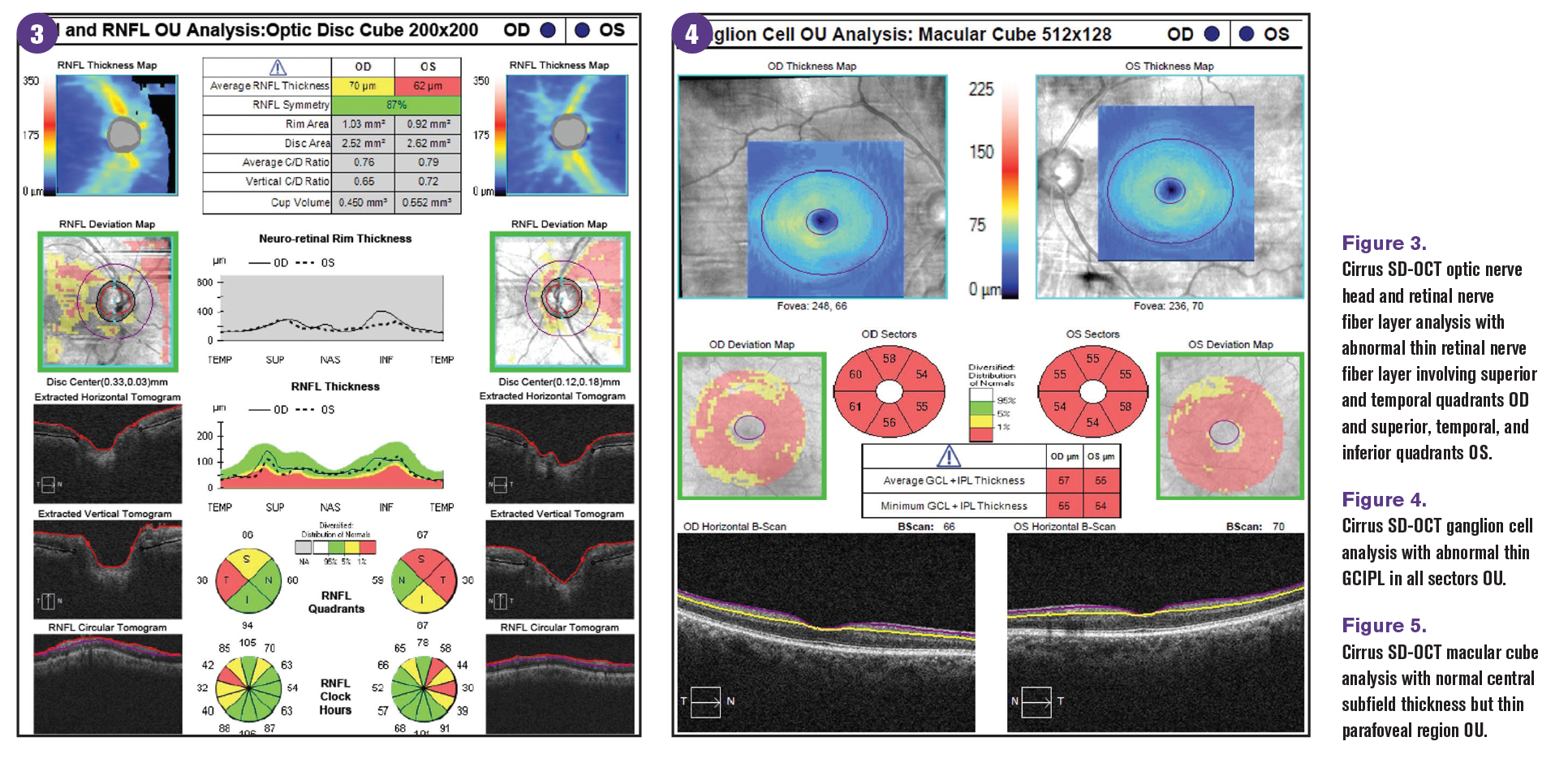
OCT is used in glaucoma evaluation to image structural damage from glaucomatous optic neuropathy. However, thinning of the pRNFL and ganglion cell layer (GCL) thickness is not specific to glaucoma. An overlap of OCT findings from DRN exists that can confound the assessment of glaucoma. Results of studies evaluating pRNFL in patients with diabetes have identified a reduction in pRNFL thickness compared with age-matched norms even in patients without active diabetic retinopathy.20,21
In a meta-analysis of studies examining pRNFL of patients with preclinical diabetic retinopathy, pRNFL thinning was more extensive in the superior and inferior quadrants than the nasal and temporal quadrants, similar to that seen in patients with glaucoma.12 Superotemporal retinal nerve fiber layer defects have also been identified in patients with diabetes with or without active diabetic retinopathy and are believed to be secondary to DRN.20
In addition, the normative data in OCT instruments excludes patients with diabetes or active diabetic retinopathy. Therefore, when evaluating OCT pRNFL thickness in patients with diabetes, extra caution should be taken when comparing to the instrument’s color-coded normative database.
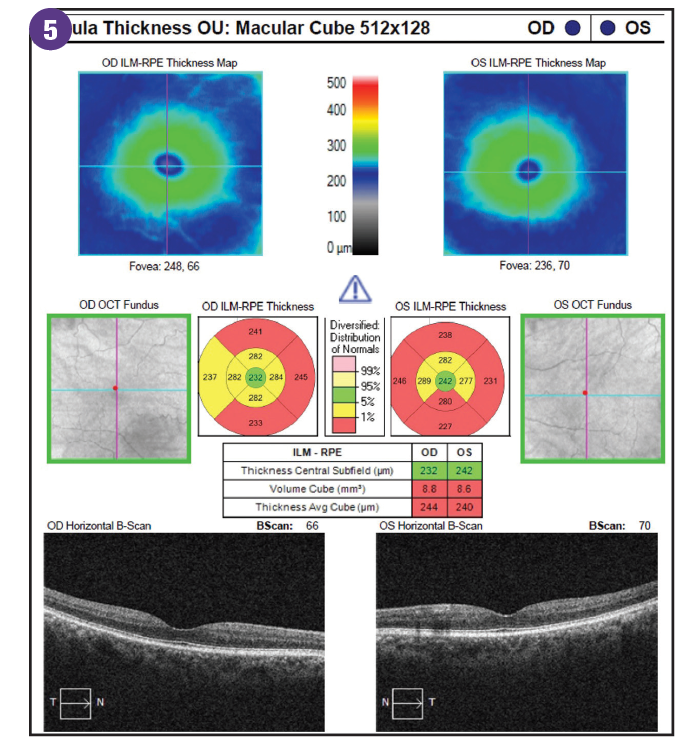
Ganglion cell analysis has been found to be comparable to pRNFL in the diagnosis of glaucoma.22 OCT scans concentrate on the macular GCL thickness because the macula has the highest density of retinal ganglion cells.23 However, diabetes can damage the macula from diabetic macular edema (DME) and macular ischemia. Similar to pRNFL thickness in patients with diabetes, the GCL is thinner in those with diabetes compared with healthy controls even in the absence of diabetic retinopathy.24-25 The early damage to the neural retina from DRN is independent of, and occurs before, diabetic vascular changes. The pattern of ganglion cell-inner plexiform layer (GCIPL) loss from diabetes has been found to be diffuse and involves all sectors.25
Newer technology such as OCT-angiography (OCTA) provides insight on structural and angiographic changes in diabetes and glaucoma. Evaluation of peripapillary capillary density, superficial capillary plexus density, and deep capillary plexus density reveals no differences in the 2 conditions because both lead to significant capillary density loss. This overlap of OCTA findings of glaucoma and diabetes demonstrates that although the mechanisms of inciting injury on the retinal ganglion cells are different, the pathologic process results in similar modes of neuroretinal inflammation and degeneration.26
Treatment considerations
Treatment of diabetic retinopathy primarily includes laser photocoagulation and anti-vascular endothelial growth factors (anti- VEGF). Conventional panretinal photocoagulation (PRP) for the treatment of proliferative diabetic retinopathy (PDR) has been found to cause an increase in pRNFL and macular GCL thickness initially after treatment because of inflammation and edema. The upregulation and expression of VEGF after PRP likely contributes to not only an increase in macular GCL thickness, but also overall macular thickness.27,28 An increase in pRNFL and macular GCL thickness can mask underlying glaucomatous damage. However, months after PRP treatment a subsequent decrease in pRNFL and macular GCL thickness occurs because of apoptosis from continuous low-grade inflammation or damage to the retinal layer from dissipation of laser energy and consequent thermal damage.27
Results from studies have shown the reduction in pRNFL thickness can continue up to 3 years post PRP.29 However, newer lasers such as pattern scanning laser or Pascal system have less impact on pRNFL and macula thickness because of less thermal damage to the retinal layers.30-31
Anti-VEGF intravitreal injections have become the treatment of choice for center-involving DME and play a role in the management of PDR.32,33 However, it has been proposed that VEGF-A has neuroprotective, neurotrophic, and neurodevelopmental properties. Therefore, the use of anti-VEGF may exacerbate retinal ganglion cell death, especially in eyes at risk for or with glaucomatous damage.34
Additionally, repeated intravitreal injections of anti-VEGF have been found to cause sustained elevation of IOP in 6% of patients. This is believed to be an immunological reaction related to inflammation and can significantly impact patients with glaucoma and increase the risk for progression and their management of IOP control.35
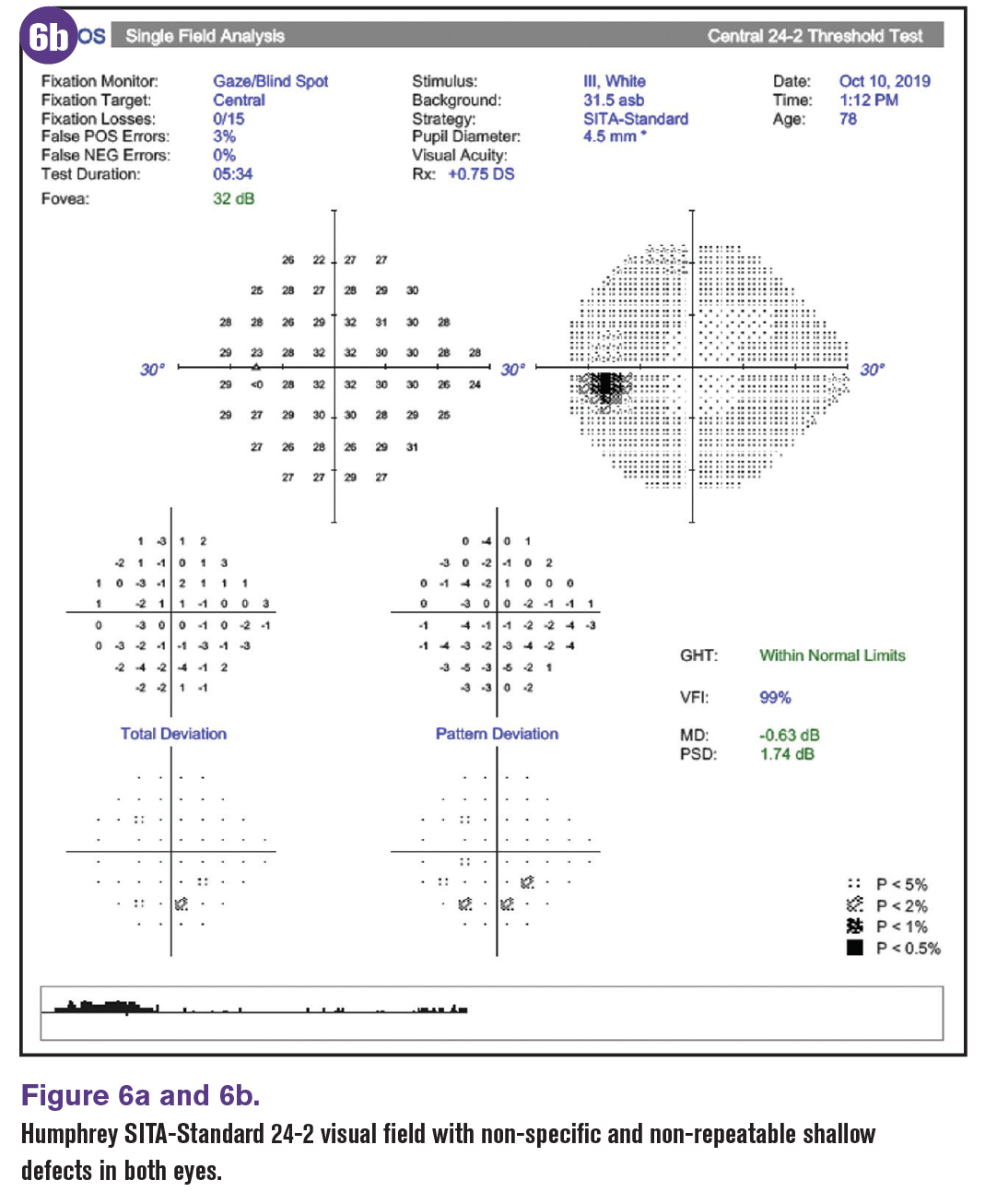
Conclusion
The relationship of diabetes and glaucoma is complex. Laboratory studies and the pathophysiology of the 2 conditions suggest they are strongly related. The mechanism of diabetes-induced neurodegeneration and glaucoma lead to similar pathologic processes that may be difficult to differentiate clinically. In addition, the treatment of diabetic retinopathy may impact and confound the assessment for glaucoma.
Further studies are needed to better understand the direct relationship of the 2 conditions. Clinicians should consider the impact of diabetes on the assessment and management of their patients with glaucoma.

References
1. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. 2020.
2. Wong VHY, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom. 2011;94(1):4-23. doi:10.1111/j.1444- 0938.2010.00546.x
3. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi:10.1016/j.ophtha.2014.05.013
4. Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994;101(7):1173-1177. doi:10.1016/ s0161-6420(94)31191-2
5. Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(4):712-718. doi:10.1016/s0161-6420(97)30247-4
6. Chopra V, Varma R, Francis BA, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma. The Los Angeles Latino Eye Study. Ophthalmology. 2008;115(2):227-232e1. doi:10.1016/j. ophtha.2007.04.049
7. Tielsch JM, Katz J, Quigley HA, Javitt JC, Sommer A. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey.
8. Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113(7):918-924. doi:10.1001/ archopht.1995.01100070092031
9. Wang YX, Hu LN, Yang H, Jonas JB, Xu L. Frequency and associated factors of structural progression of open-angle glaucoma in the Beijing Eye Study. Br J Ophthalmol. 2012;96(6):811-815. doi:10.1136/bjophthalmol-2011-301224
10. Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci. 2016;113(19):E2655-2664. doi:10.1073/ pnas.1522014113
11. Lunch SK, Abramoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vision Res. 2017;139:101-107. doi:10.1016/j. visres.2017.03.003
12. Chen X, Nie C, Gong Y, et al. Peripapillary retinal nerve fiber layer changes in preclinical diabetic retinopathy: a meta-analysis. PLoS ONE. 2015;10(5):e0125919. doi:10.1371/journal. pone.0125919
13. Lim HB, Shin YI, Lee MW, Park GS, Kim JY. Longitudinal changes in the peripapillary retinal nerve fiber layer thickness of patients with type 2 diabetes. JAMA Ophthalmol. 2019;137(10):1125- 1132. doi:10.1001/jamaophthalmol.2019.2537
14. Dielemans I, de Jong PT, Stolk R, et al. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology. 1996;103(8):1271-1275. doi:10.1016/s0161- 6420(96)30511-3
15. Ramm L, Herber R, Spoerl E, Pillunat LE, Terai N. Intraocular pressure measurements in diabetes mellitus. Eur J Ophthalmol. 2020;30(6):1432-1439. doi:10.1177/1120672119890517
16. Kumar N, Pop-Busui R, Musch DC, et al. Central corneal thickness increase due to stromal thickening with diabetic peripheral neuropathy severity. Cornea. 2018;37(9):1138-1142. doi:10.1097/ICO.0000000000001668
17. Bao YK, Yan Y, Gordon M, McGill JB, Kass M, Rajagopal R. Visual field loss in patients with diabetes in the absence of clinically-detectable vascular retinopathy in a national representative survey. Invest Ophthalmol Vis Sci. 2019;60(14):4711-4716. doi:10.1167/iovs.19- 28063
18. Zico OA, El-Shazly AA, Ahmed-Hamid Ahmed EE. Short wavelength automated perimetry can detect visual field changes in diabetic patients without retinopathy. Indian J Ophthalmol. 2014;62(4):383-387. doi:10.4103/0301- 4738.126986
19. Han Y, Adams AJ, Bearse MA, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eye with little or no retinopathy. Arch Ophthalmol 2004;122(12):1809-1815. doi:10.1001/ archopht.122.12.1809
20. Jeon SJ, Kwon JW, La TY, Park CK, Choi JA. Characteristic of retinal nerve fiber layer defect in nonglaucomatous eyes with type II diabetes. Invest Ophthalmol Vis Sci. 2016;57(10):4008-4015. doi:10.1167/iovs.16-19525
21. Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005:219(6):379-385. doi:10.1159/000088382
22. Kotowski J, Folio LS, Wollstein G, et al. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol. 2012;96(11):1420-1425. doi:10.1136/ bjophthalmol-2011-301021
23. Hood DC, Raza AS, Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1-21. doi:10.1016/j. preteyeres.2012.08.003
24. Carpineto P, Toto L, Aloia R, et al. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye (Lond). 2016;30(5):673-679. doi:10.1038/eye.2016.13
25. Chhablani J, Sharma A, Goud A, et al. Neurodegeneration in type 2 diabetes: evidence from spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(11):6333-6338. doi:10.1167/iovs.15- 17334
26. Spaide RF. Measurable aspects of the retinal neurovascular unit in diabetes, glaucoma, and controls. Am J Ophthalmol. 2019;207:395-409. doi:10.1016/j.ajo.2019.04.035
27. Kim JJ, Im JC, Shin JP, Kim IT, Park DH. One-year follow-up of macular ganglion cell layer and peripapillary retinal nerve fibre layer thickness changes after panretinal photocoagulation. Br J Ophthalmol. 2014;98(2):213-217. doi:10.1136/ bjophthalmol-2013-304349
28. Mukhtar A, Khan MS, Junejo M, lIshaq M, Akbar B. Effect of panretinal photocoagulation on central macular thickness and visual acuity in proliferative diabetic retinopathy. Pak J Med Sci. 2016;32(1):221-224. doi:10.12669/ pjms.321.8758
29. Wadhwani M, Bhartiya S, Upadhaya A, Manika Ml. A meta-analysis to study the effect of panretinal photocoagulation on retinal nerve fiber layer thickness in diabetic retinopathy patients. Rom J Ophthalmol. 2020;64(1):8-14.
30. Muquit MM, Young LB, McKenzie R, et al. Pilot randomized clinical trial of Pascal TargETEd retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol. 2013;97(2):220-227. doi:10.1136/ bjophthalmol-2012-302189
31. Lee DE, Lee JH, Lim HW, Kang MH, Cho HY, Seong M. The effect of pattern scan laser photocoagulation on peripapillary retinal nerve fiber layer thickness and optic nerve morphology in diabetic retinopathy. Korean J Ophthalmol. 2014;28(5):408-416. doi:10.3341/ kjo.2014.28.5.408
32. Diabetic Retinopathy Clinical Research Network; Elman MJ, Qin H, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312-2318. doi:10.1016/j. ophtha.2012.08.022
33. Writing Committee for the Diabetic Retinopathy Clinical Research N, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical Trial. JAMA. 2015;314(20):2137-2146. doi:10.1001/ jama.2015.15217
34. Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol 213;182(4):1379- 1390. doi:10.1016/j.ajpath.2012.12.032
35. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95(8):1111-1114. doi:10.1136/bjo.2010.180729

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.