Treatment options for ocular allergies
Clinicians can offer patients both prescription and OTC options
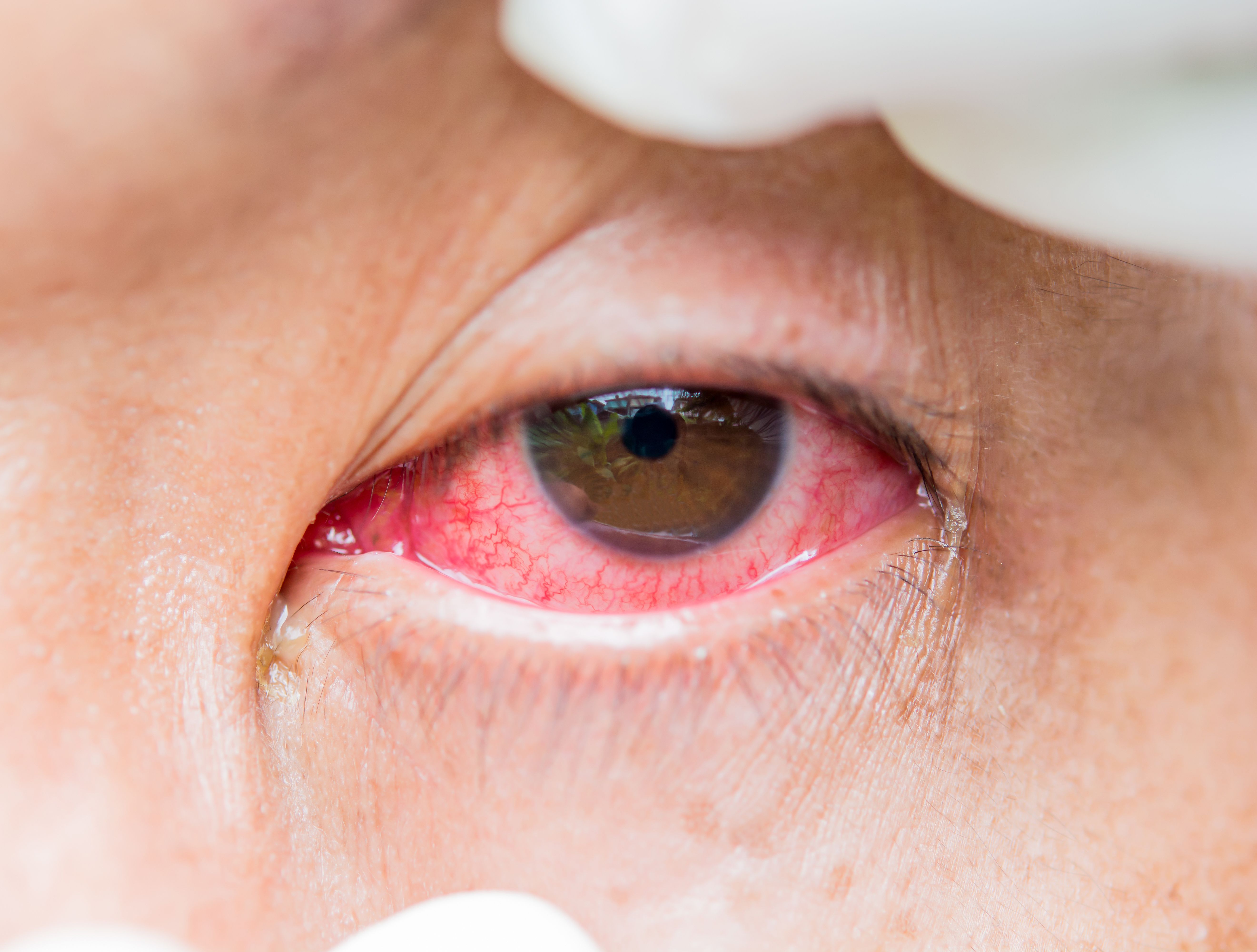

Ocular allergies are among the most common conditions seen in clinic by optometrists and ophthalmologists. The more common and less severe types of allergic conjunctivitis are seasonal and perennial allergic conjunctivitis, while the more severe types are atopic keratoconjunctivitis, vernal keratoconjunctivitis, and giant papillary conjunctivitis.1
Eye care practitioners should stay up to date on prescription and OTC treatment options to best manage patients. This article aims to provide an overview of currently available treatment options and those under investigation.
Allergic conjunctivitis
Seasonal and perennial allergic conjunctivitis are type 1 hypersensitivity reactions that result in itching, tearing, eyelid and bulbar injection, and photophobia.2 First-line treatment options include avoidance of the allergen, cool compresses, and preservative-free artificial tears. A refrigerated and nonviscous artificial tear formulation is often soothing to the patient while rinsing the allergens from the ocular surface. Topical antihistamines, mast cell stabilizers, combination antihistamine/mast cell stabilizers, and steroid medications are also effective treatment options to reduce symptoms.3
Antihistamines
Three H1 receptor antagonists available by prescription only are bepotastine besilate (Bepreve; Bausch + Lomb), emedastine difumarate (Emadine; Alcon) and alcaftadine (Lastacaft; Allergan). They are indicated for itching associated with allergic conjunctivitis and typically dosed twice a day.
Recently, the FDA approved cetirizine (Zerviate; Eyevance Pharmaceuticals) for topical use. Cetirizine has been around for years as the OTC oral drug Zyrtec. Clinical trials confirmed Zerviate is effective at reducing chemosis, eyelid swelling, tearing, redness, and nasal symptoms. It has a rapid onset of action, with improvement of allergy signs and symptoms within 15 minutes.4
Mast cell stabilizers
Mast cell stabilizers are available by prescription as cromolyn sodium (Crolom; Bausch + Lomb) and nedocromil (Alocril; Allergan). They act by preventing the degranulation of mast cells, thereby inhibiting release of histamines and cytokines that cause the symptoms associated with allergic conjunctivitis.5 They are infrequently used as monotherapy and most often used for chronic perennial allergic conjunctivitis or prevention of symptoms in anticipated seasonal allergic conjunctivitis.
Combination antihistamine and mast cell stabilizer
Combination antihistamine and mast cell stabilizer ophthalmic solutions are an excellent option for patients who already exhibit symptoms associated with allergic conjunctivitis. The antihistamine works quickly to reduce symptoms of itching while the mast cell stabilizer works to prevent symptoms for a longer period of time.
Many formulations are available OTC, including ketotifen (Zaditor; Alcon, and Alaway; Bausch + Lomb) and olopatadine (Pataday; Alcon). Zaditor was the first combination ophthalmic drop available to purchase OTC. Prior to 2 years ago, olopatadine was available only in prescription form at various concentrations, including 0.1% olopatadine (Patanol), 0.2% olopatadine (Pataday), and 0.7% olopatadine (Pazeo). Alaway is now available in a preservative-free formulation for patients with ocular allergy who may also experience sensitivity to preservatives commonly found in ophthalmic drops.
Topical steroids
Topical steroids are useful for patients with severe allergic conjunctivitis as well as those who do not respond to antihistamines or mast cell stabilizers. Some patients may require a short course of steroids to reduce inflammation and provide comfort while starting chronic therapy.
Loteprednol etabonate 0.2% (Alrex; Bausch + Lomb) is a topical corticosteroid indicated for the signs and symptoms of seasonal allergic conjunctivitis. Alrex is a low-concentration, ester-based steroid that shows to be an effective treatment with a good safety profile. Clinical study results indicated only 1% of patients had a clinically significant increase in intraocular pressure (10 mm Hg or higher).6
Future treatment options
Johnson & Johnson Vision recently announced approval for a contact lens indicated for allergic eye itch in Japan and Canada. The technology combines a daily disposable contact lens (etafilcon A) with the antihistamine ketotifen. It is not available in the United States; however, the company is working with regulatory agencies to expand its availability.
Aldeyra Therapeutics is currently undergoing phase 3 clinical investigation of reproxalap, a small-molecule biologic inhibitor of RASP or reactive aldehyde species. Specifically, the study is considering the efficacy of 0.25% ophthalmic solution for the treatment of dry eye disease and allergic conjunctivitis.7
Conclusion
Various OTC and prescription treatment options are available for allergic conjunctivitis. The best treatment option varies on a case-by-case basis pending clinical presentation and severity.
At present, current treatment options are limited for contact lens wearers and patients with concomitant dry eye disease; however, there are potential treatments in the pipeline for these scenarios. Stay tuned.

References
1. La Rosa M, Lionetti E, Reibaldi M, et al. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013;39:18. doi:10.1186/1824-7288-39-18
2. Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115(1):118-22. doi:10.1016/j. jaci.2004.10.042
3. Mounsey AL, Gray RE. Topical antihistamines and mast cell stabilizers for treating allergic conjunctivitis. Am Fam Physician. 2016;93(11):915-916.
4. Meier EJ, Torkildsen GL, Gomes PJ, Jasek MC. Phase III trials examining the efficacy of cetirizine ophthalmic solution 0.24% compared to vehicle for the treatment of allergic conjunctivitis in the conjunctival allergen challenge model. Clin Ophthalmol. 2018;12:2617-2628. doi:10.2147/OPTH. S185835
5. Finn DF, Walsh JJ. Twenty-first century mast cell stabilizers. Br J Pharmacol. 2013;170(1):23-37. doi:10.1111/bph.12138
6. Shulman DG, Lothringer LL, Rubin JM, et al. A randomized, double-masked, placebo-controlled parallel study of loteprednol etabonate 0.2% in patients with seasonal allergic conjunctivitis. Ophthalmology. 1999;106(2):362-369. doi:10.1016/S0161-6420(99)90077-5
7. Aldeyra Therapeutics announces positive top-line symptom and sign results from run-in cohort of phase 3 TRANQUILITY trial in dry eye disease. News release. January 7, 2021. Accessed July 6, 2021. https://ir.aldeyra.com/news-releases/ news-release-details/aldeyra-therapeutics-announces-positive-top-line-symptom-and

Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.