This week in optometry news

Catch up on some of the eyecare headlines from this past week!
This week in optometry news: September 23, 2019Vision Expo West roundup neurolens introduces new offerings, appoints VP and CMO
neurolens introduced new offerings to provide more successful outcomes for private-practice optometry.
The new streamlined software improves the neurolens Measurement Device’s eye tracking ability, which will increase patient outcomes.
neurolens will roll-out patient financing based on a partnership with Affirm, whose customers include Peloton and RING. The partnership will offer credit to allow more consumers to experience the neurolens’ software, at no cost to eyecare practioners.
neurolens has appointed Matt Swartz as vice president of sales and business development
Swartz will be responsible for the growth and expansion of sales territories, developing the sales team in order to increase the value proposition to the iECP, and forming strategic alliances and partnerships.
Prior to joining neurolens, Swartz served as senior director of strategic accounts for Essilor of America, Essilux.
neurolens has also appointed Pierre Bertrand as its new chief marketing officer.
Prior to joining neurolens, Bertrand served as president of Vision Associates for Essilor, Inc. During his eight-year tenure at Essilor, Bertrand held executive leadership positions as vice president of marketing for Essilor of America and president of Essilor Canada. Bertrand also held positions at Pfizer, including as regional marketing director.

Top story: 10 eyecare apps for more efficient patient care
Check out these 10 mobile app recommendations to help ODs manage and provide a faster, quality service to their patients.
Read more! CooperVision celebrates OptiExpert app, introduces new sustainability exhibit booths
In the 10 months since its U.S. launch, CooperVision’s OptiExpert app has been adopted by thousands of eyecare practitioners.
The U.S. has become the third-largest OptiExpert market globally, behind only the U.K. and Germany, which were among the first countries to introduce the app in 2014.
The digital tool-which includes CooperVision multifocal and toric contact lens calculators, and oxygen profiles that display the oxygen transmissibility of a select range of lenses-has logged more than 25,000 new users and garnered over 7,400 app downloads in the U.S. since being launched December 2018.
Nearly 60 percent of users utilize the desktop version of the tool.
OptiExpert includes diagnostic lens recommendations from CooperVision product families, including clarito 1 day, MyDay, Biofinity, Avaira Vitality, and Proclear.
Sustainability efforts
CooperVision is also continuing its initiative to minimize environmental impact areas of business.
From Leadership in Energy and Environmental (LEED) silver-certified manufacturing and distribution facilities in Costa Rica and Spain to 100-percent renewable electricity usage in Rochester, N.Y., the company is making efforts to save water, conserve energy, reduce, reuse, and recycle resources
CooperVision’s booth at Vision Expo West exhibited sustainable features such as:
Reusable or reused elements that will be used for future trade shows. These elements included a sink, plumbing components, and fit-set trays.
The shipping materials used to create the booth are also reusable, as most of the booth elements were packed and transported in crates. In doing so, the need for wasteful materials-such as shrink wrap and banding used to secure skidded goods, which is discarded after each use-is further minimized.
To further reduce waste and consumption of paper-based materials in graphic print and production, CooperVision opted to transition the majority of its in-booth messaging to digital displays.
The monitors-and other heavy equipment-are also rented at each show; the use of shared resources means the company no longer needs to upgrade the technology every few years, and is environmentally beneficial due to a lack of need for them to not be shipped from city to city
Recyclable
Booth materials do have a limited lifespan, so CooperVision selected recyclable products including flooring, seating, and overhead fabric graphics.
Energy efficient
Canopy lights utilize low-voltage LED bulbs to minimize the energy needed to illuminate the booth.
Lightweight shipping
The plywood used to fabricate the internal structures of CooperVision’s booth is 30 percent lighter than traditional sheets of plywood.
Traditionally, the overhead displays at trade shows require full-truss systems, which are heavy and add to shipping costs. CooperVision’s new hanging structures-including the overhead fabric graphics, which utilizes aluminum tubing framework instead of a truss system-are lightweight, easily broken down and packed into a low profile to reduce their footprint for transit.
Sourced responsibly
The booth’s maple paneling and high-pressure laminates were sourced from vendors focused on sustainably and sourcing materials.
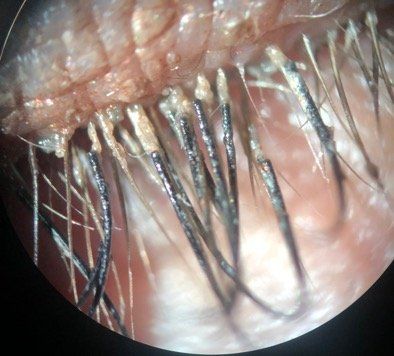
Top story: A case of demodex infestation with eyelash extensions
Patients with eyelash extensions face an increased risk for developing Demodex. Learn why correctly identifying this infestation, as well as proper patient education on treatment and management, are keys to a successful outcome.
Learn more! Oasis introduces new line of dietary supplements

Oasis Medical Inc. unveiled its new dietary supplements, Oasis Tears Vision and Oasis Tears Omega at Vision Expo West.
Oasis Tears Vision is the first of Oasis' supplements to be brought to market this year. Oasis Tears Omega-3 dietary supplements will also be available later this year.
The supplements are a patent-pending formulation delivered in a small soft gel. It will be packaged as a 30-day supply.
This week in optometry news: Sept. 16, 2019
BCLA, AIO partner with GOC to combat illegal contact lens sales in UK
The Association for Independent Optometrists and Dispensing Opticians (AIO) and the British Contact Lens Association (BCLA) have joined together in an initiative to work closely with the General Optical Council (GOC) to end the illegal sale of contact lenses across the UK.
While the GOC already has a London-based team investigating complaints about illegal contact lens sales, the organization does not have a physical presence across the country.
The intention is for AIO and BCLA members to become the ‘eyes and ears’ on the High Street and identify UK-based online suppliers to ensure early investigations and proper enforcement action, taken in association with Local Trading Standards Officers, is fully effective.
In the coming months AIO and BCLA will report on the impact of the initiative on halting illegal sales.
For more details, visit www.bcla.org.uk.

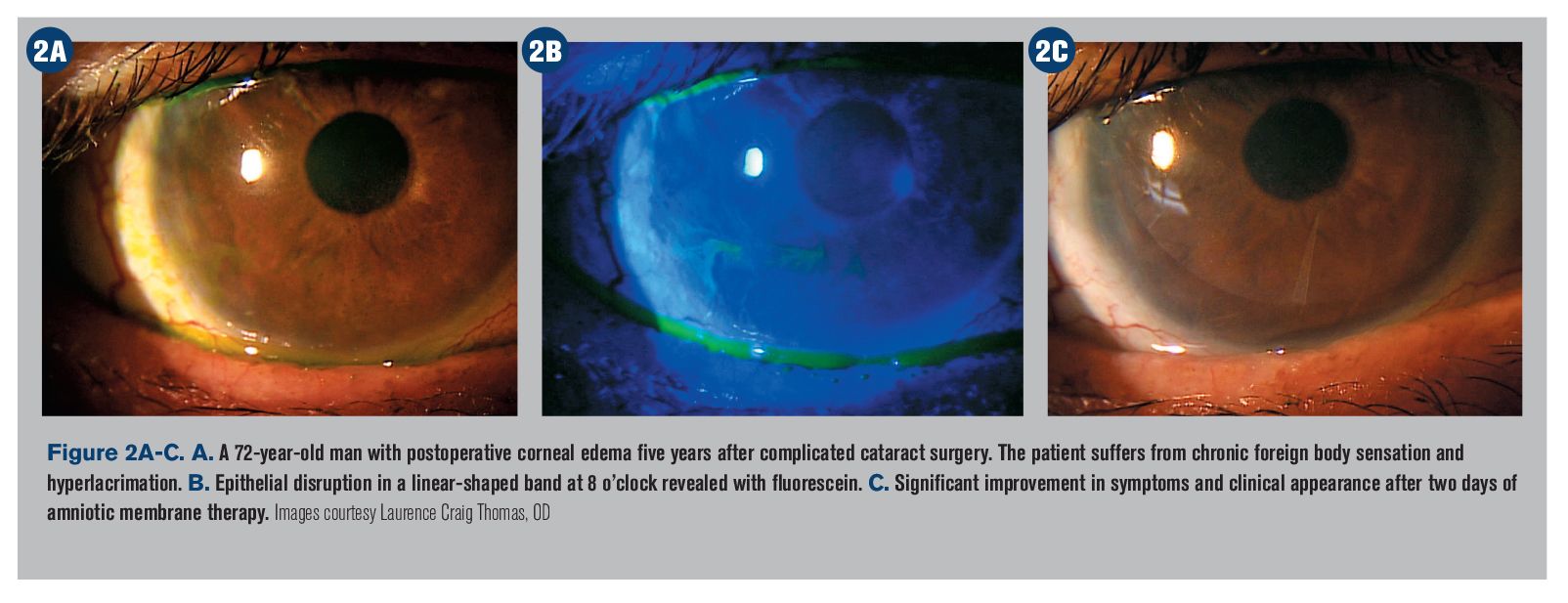
Top story: 3 updates to treating Sjögren’s syndrome and dry eye
Take a look at the latest and most important updates in the treatment for Sjögren’s syndrome and dry eyes, such as new guidelines, a potential new medication, and another glance at the Sjögren’s biomarker test.
Read here!PhysIOL to launch FineVision Triumf for presbyopia correction

Beaver-Visitec International (BVI), through its PhysIOL subsidiary, has announced the European launch of FineVision Triumf, an extended depth-of-focus (EDOF) trifocal presbyopia correcting intraocular lens (IOL) for patients undergoing cataract surgery.
Results from a year of clinical studies will be presented at the European Society for Cataract and Refractive Surgeons (ESCRS), held September 14-17, in Paris.
FineVision Triumf combines trifocal technology and EDOF optics with the goal of reducing low-light condition side effects, which result in glare and halos for some patients after implantation of trifocal IOLs.
These effects are the result of longitudinal chromatic aberration (LCA). FineVision Triumf is free of LCA at far and intermediate focus points, resultling in higher contrast sensitivity, less risk of photic phenomena, and an improved quality of vision in low-light conditions, according to the company.
The FineVision Triumf lens is available in Europe and certain international markets. The company intends to make the technology available in the U.S., China, and other markets outside Europe in the near future.

Popular video: How to start incorporating dry eye in your practice
Scott Schachter, OD, shares two tried-and-true approaches for ODs looking to start providing dry eye services in their practices.
Watch hereProQR receives fast track designation from FDA for QR-1123

ProQR Therapeutics N.V. announced that it received fast track designation from the U.S. Food and Drug Administration (FDA) for QR-1123.
QR-1123 is a first-in-class investigational antisense oligonucleotide designed to address the underlying cause of vision loss associated with autosomal dominant retinitis pigmentosa (adRP) due to the P23H mutation in the rhodopsin (RHO) gene.
Fast Track designation is granted by the FDA to drugs in development for serious conditions with the potential to fulfill an unmet medical need. It was established with the intention to bring promising drugs to patients sooner by facilitating development with more frequent FDA interactions and expediting the review process.
With the fast track designation, enrollment in the Phase 1/2 (Aurora) clinical trial for QR-1123 is expected to begin in the coming months, according to the company.

Popular story: Explore the relationship between dry eye and sleep
Chronic sleep problems affect 70 million adults in the U.S. Find out why a restless night of sleep may be to blame for the development of DED in your patients.
Find out!
UK, Spain CooperVision locations earn environmental certifications
Two newly-constructed CooperVision sites in Europe have been awarded certifications for sustainable design and operation.
The company’s secondary packaging and distribution facility in Southampton, England, has earned a Building Research Establishment Environmental Assessment Method (BREEAM) “excellent” rating.
The site incorporates high-efficiency light-emitting diode (LED) lighting as well natural light. The facility also encourages electric car use with integrated charge points.
The company’s new distribution center in Madrid, Spain, has earned Leadership in Energy and Environmental Design (LEED) certification.
The warehouse features intelligent LED and natural lighting as well as on-site solar power generation to meet a portion of its energy needs. It also recycles 100 percent of cardboard, plastic, and wood pallets, and is located near public transit options.
The Madrid site also includes CooperVision’s Iberia Center of Innovation.
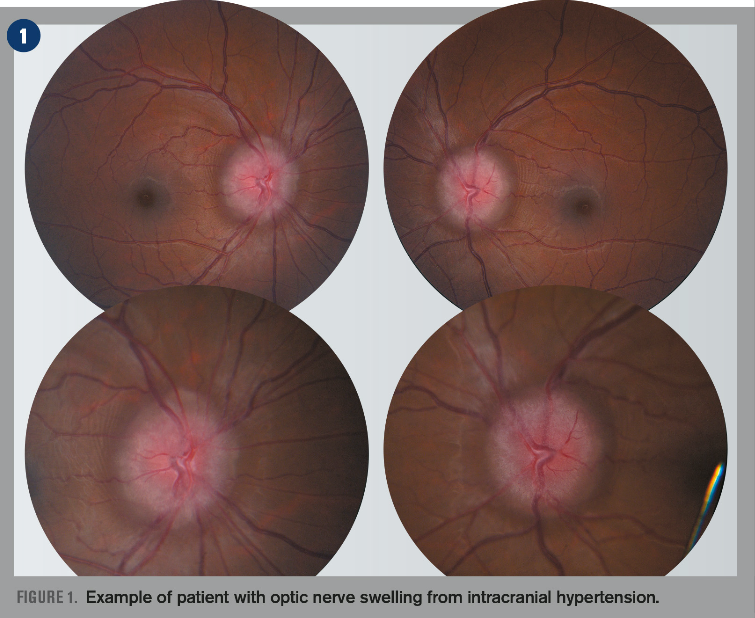
Popular story: How to diagnose a swollen optic nerve
There is not much more intimidating for an OD during a normal clinic day than for a patient to come in complaining of vision loss and the dilated fundus exams reveals a swollen optic nerve(s). Find out how to work through the differential diagnosis
Find out!
BCLA calls for papers ahead of NCC 2020
The British Contact Lens Association (BCLA) is issuing a call for papers ahead of the annual collaborative research symposium to be held in the Netherlands next year.
The Netherlands Contact Lens Congress (NCC) and BCLA’s collaborative research symposium is being run as part of the NCC 2020, scheduled for March 15 and 16 in Eindhoven, Netherlands.
The symposium is designed to showcase clinically relevant research from around the globe. New researchers, clinicians, and young academics are encouraged to present at this level.
Symposium submission should be in the form of abstracts and can be in paper or digital poster (to be allocated by the Academic Committee). Case reports are also encouraged to be submitted.
The theme for NCC 2020 is “Beyond 2020.” Practitioners are encouraged to contribute to the science stream and submit case reports.
All submissions must be made using the online submissions form, accessible via the BCLA website.
A pre-screening service will be available until September 25 ahead the November 1 deadline for final submissions.
The company expects to use the remainder of the net proceeds for general corporate purposes, further development of other potential pipeline opportunities, evaluating possible uses of its existing proprietary portfolio of molecules beyond ophthalmology, external business development efforts, and its manufacturing activities, including the operation of its own manufacturing plant in Ireland.

Popular story: Consider outsourcing opportunities in your practice
Outsourcing is a popular concept to help grow ODs’ businesses. Less stress and a happier work environment can translate to better patient care. Find out why it can be a win-win situation for staff as well as ODs.
Find out!Aerie Pharma secures additional $41.2M in convertible debt funding
Aerie Pharmaceuticals, Inc. announced that the initial purchasers of the previously announced offering of Aerie’s 1.50% Convertible Senior Notes have elected to fully exercise their option to purchase an additional $41.25 million aggregate principal amount of the notes.
Following the sale closing, there will be a total of $316.25 million aggregate principal amount of the notes outstanding.
Aerie intends to use approximately $4.3 million of the net proceeds from the sale of additional notes pursuant to the option to pay the cost of additional capped call transactions.
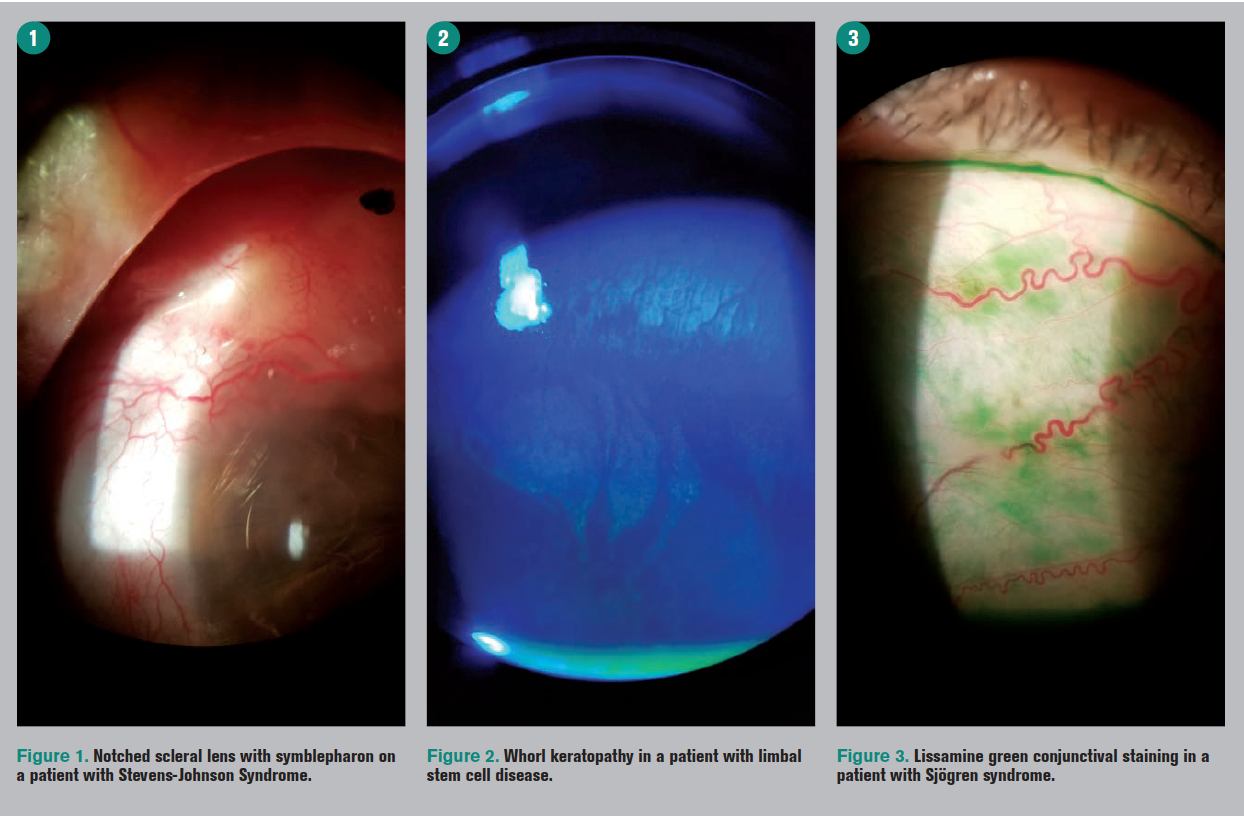
Popular story: Scleral contact lenses help manage ocular surface disease
Many patients with corneal and ocular surface conditions also experience dry eye. Find out how scleral lenses can help to provide good vision, comfort, and ocular health.
Find out!AOA announces 2019 Resident Travel Fellowship recipients

The American Academy of Optometry (AAO) has released the names of the 2019 recipients of the 2019 Resident Travel Fellowship Awards.
The travel fellowships will allow residents to attend Academy 2019 Orlando and 3rd World Congress of Optometry, scheduled for Oct. 23-27.
The recipients are as follows:
Allergan Resident Travel FellowshipsSponsored by Allergan
Dana Rhea, OD, Northeastern State University
Corinne Wong, OD, Southern California College of Optometry
Mary Chivetta, OD, University of Missouri at St. Louis
Luke Lirones, OD, Michigan College of Optometry at Ferris State University
Nicole Harris, OD, Illinois College of Optometry
Elizabeth Davis, OD, Southern College of Optometry
Allison Choi, OD, Pacific University
Vincent Chan, OD, Pacific University
Jesal Haribhakti, OD, Indiana University
Sara Moses, OD, University of Alabama at Birmingham
Amrit Bilkhu, OD, University of California, Berkeley
Courtney Cape, OD, University of Houston
Abigail Gonsalves, OD, Indiana University
Courtney Hongo, OD, Southern California College of Optometry
Dennis Giang, OD, Southern California College of Optometry
Allegra Burgher, OD, Southern California College of Optometry
Skylar Williams, OD, University of Houston
Lucinda Kauffman, OD, Pennsylvania College of Optometry
Abigail Strauss, OD, Illinois College of Optometry
Carissa Hintz, OD, Pacific University
Jessica Jankiewicz, OD, Illinois College of Optometry
Madison Goodfellow, OD, Illinois College of Optometry
Kelly Morgan, OD, MS, The Ohio State University
Sydney Cooper, OD, Southern College of Optometry
Maria Adriaansen, OD, Pennsylvania College of Optometry
Elizabeth Brooks, OD, Pennsylvania College of Optometry
Vikarma Brooks, OD, Pennsylvania College of Optometry
Andrew Henderson, OD, Northeastern State University
Daisy Berisha, OD, State University of New York
Branden Shaffer, OD, Indiana University
Sohail Sakkari, OD, State University of New York
Rachel Choi, OD, University of Houston
Kimber Kenzli, OD, Pacific University
Lauren Fernandez, OD, Southern California College of Optometry
Reid Gardner, OD, Southern California College of Optometry
Sarah Quan, OD, PhD, State University of New York
Larissa Krenk, OD, Indiana University
Jaana Ashtiani-Zarandi, OD, Michigan College of Optometry at Ferris State University
Jimmy Nguyen, OD, Nova Southeastern University
Taylor Phillips, OD, MBA, University of Alabama at Birmingham
Anterior Segment Resident Travel FellowshipsSponsored by the Anterior Segment Section of the AAO
Brittany Hoyle, OD, Southern California College of Optometry
Paige Sorrentino, OD, Midwestern University Arizona College of Optometry
Arizona Chapter Resident Travel FellowshipSponsored by the Arizona Chapter of the American Academy of Optometry
Jacqueline Yi, OD, Sierra Vista VA Community Based Outpatient Clinic
Cornea, Contact Lenses and Refractive Technologies Resident Travel FellowshipsSponsored by CooperVision
Nicole Poon, OD, Pennsylvania College of Optometry
Christopher Albright, OD, Michigan College of Optometry at Ferris State University
Thanhan Nguyen, OD, Illinois College of Optometry
Audrey Janelle-Brousseau, OD, University of Montreal
Baljinder Momrath, OD, Pennsylvania College of Optometry
Vanessa Wang, OD, State University of New York
Chelsea Bradley, OD, University of California, Berkeley
Ryan Rutschilling, OD, The Ohio State University
Mari Fujimoto, OD, Pacific University
Pooja Mahadev, OD, Nova Southeastern University
Valerie Lim, OD, Southern California College of Optometry
Christina Wenn, OD, University of Houston
Duc Tran, OD, University of the Incarnate Word
Ghazal Naseri, OD, University of California, Berkeley
Joseph Isik, OD, Illinois College of Optometry
Amrit Jawanda, OD, New England College of Optometry
Jacqueline Benoit, OD, The Ohio State University
Kimberly Weisenberger, OD, The Ohio State University
Haley Italia, OD, New England College of Optometry
Kendra Phillis, OD, New England College of Optometry
Rosa (Yawen) Yang, OD, University of Waterloo
Kevan Smith, OD, Southern College of Optometry
Jessica Sun, OD, Southern California College of Optometry
Matthew Lee, OD, University of Missouri at St. Louis
Florida Chapter Resident Travel FellowshipsSponsored by the Florida Chapter of the American Academy of Optometry
Alexandria Rawls, OD, Bay Pines VA Medical Center
Mickinzee Combs, OD, Nova Southeastern University
Lauren Nicholas, OD, Nova Southeastern University
Esther Park, OD, Nova Southeastern University
IKA Resident Travel FellowshipsSponsored by the International Keratoconus Academy
Sophia Leung, OD, Northeastern State University
Yue Yu, OD, PhD, New England College of OptometryThis week in optometry news: September 2, 2019
Essilor Vision Foundation encourages patients to take 20/20 vision pledge
Essilor Vision Foundation (EVF) and Essilor of America are raising awareness on the need for nationwide pediatric vision care with the Essilor 20/20 Vision Pledge.
Parents are encouraged to make an online commitment to seek an eyecare professional who will prioritize their child's vision. Parents also can be entered into a sweepstakes for a chance to win a comprehensive eye exam and glasses for their child and glasses for the child's entire school (K-12).
EVF will transport the doctors, provide vision exams, and deliver the glasses to the winning school.
The Essilor 20/20 Vision Pledge sweepstakes runs through Sept. 25. The winning school is anticipated to be announced on World Sight Day on Oct. 10.

Last week’s top story: Visibly (formerly Opternative) recalls online vision test
After heavy pressure from the American Optometric Association (AOA), the online vision screening test from Visibly (formerly Opternative), is no longer available and has been recalled by the company, according to the U.S. Food & Drug Association (FDA).
Read more!
New England College of Optometry welcomes new dean of clinical affairs

Kristen Brown, OD, FAAO, has been selected to be the associate dean of Clinical Affairs at the New England College of Optometry (NECO).
Dr. Brown received her doctor of optometry from the University of California Berkeley, School of Optometry, in 1992. She completed two ocular disease residencies (from NECO and Boston University School of Medicine) and a two-year fellowship at the Pacific Cataract and Laser Center.
Dr. Brown has 27 years of experience as a clinician and educator in the industry.
She was most recently the clinical director for The Laser Center (TLC) Boston and Providence, where she managed clinical operations and maintained doctor networks.

Popular story: Modernize contact lens wear in 2019
New advances in the contact lens sphere warrant specialty fits, additional coaching, and more cost to patients. But such technology can also change a patient’s quality of life for the better.
Learn more!
CooperVision launches soft contact lens recycling program in Sweden
CooperVision has launched an initiative in Sweden offering consumers free soft contact lens recycling for all brands and manufacturers.
The CooperVision Contact Lens Recycle Program aims to reduce plastic waste by providing an easier way for wearers to recycle lenses as well as blister and foil packaging.
Wearers have the option of sending contact lens materials directly to CooperVision’s recycling partner, TerraCycle, using pre-paid mailing labels or dropping them off at a network of participating optical stores across Sweden.

Popular story: Consider outsourcing opportunities in your practice
Outsourcing is a popular concept to help grow ODs’ businesses. Less stress and a happier work environment can translate to better patient care. Find out why it can be a win-win situation for staff as well as ODs.
Find out!
UL grants InvisibleShield first-ever verification mark for HEV mitigation, true color preservation
InvisibleShield has received its first global UL-verified mark for reducing high-energy visible (HEV) blue light emissions while preserving a digital device's true screen colors.
The UL-verified mark was granted after a series of objective, science-based assessments was conducted at UL's Optical Radiation Laboratory in Raleigh-Durham, North Carolina. The tests utilized a scanning spectroradiometer with double monochromator.
For the InvisibleShield analysis, the radiance emitted from a light source before and after the application of the InvisibleShield screen protector confirmed an HEV blue light emission reduction of at least 15 percent.

Popular story: Dr. New Mom: Planning for baby and returning to work
With optometry becoming increasingly female as a profession, doctors and workplaces alike are facing the challenges of providing the best work environments and balance for new mothers. In the latest Defocus Media podcast, new mother Jennifer Lyerly, OD, talks with two other ODs who are balancing their careers as new moms.
Read and listen here!
AmblyoPlay introduces vision therapy solution for lazy eyes
AmblyoPlay has launched a software-based vision therapy solution in the U.S. market.

With AmblyoPlay, users perform vision therapy through red and blue glasses and interactive gaming software and are rewarded with tokens to exchange for physical awards sent to their homes.
The program stimulates the eye muscles through two 15-minute sessions per day.
Users have three subscription options for gaining access to AmblyoPlay: three months, six months, or a year. The different durations address the needs of the user, which can be affected by the user’s age, severity of vision problem, and how regularly he completes the vision therapy exercises.
Parents can monitor the progress of their child through AmblyoPlay’s automated progression tracker and share the results with their child’s doctor at subsequent eye examinations.
Pricing for AmblyoPlay starts at $110 for a three-month subscription The software is available for purchase on AmblyoPlay’s website for Windows and Mac computers, Android tablets, and iPads.

Popular story: Remembering the life of Dr. John Keriotis
Mike Brown, OD, MHS-CL, FAAO, takes a look at the life of his long-time friend and colleague John Keriotis, OD, and the lasting memory he leaves behind in the optometric community.
Read here!
AAO announces 2019 Student Travel Fellowship recipients
The American Academy of Optometry has announced the recipients of the 2019 Student Travel Fellowship Awards. These travel fellowships will allow students to attend Academy 2019 Orlando and 3rd World Congress of Optometry, October 23 to 27. Resident travel fellowship recipients will be announced at a later time.
The 2019 Student Travel Fellowship recipients include:
Frank W. Weymouth Student Travel Fellowship
Rebecca Deffler, OD, MS; The Ohio State University
Augustine Nti, OD; University of Houston
Irvin M. Borish Student Travel Fellowship
Nahida Akter, University of New South Wales
Abdulla Alamri, State University of New York
Brazelton Low Vision Student Travel Fellowship
Wai Fung, University of California Berkeley
Edward I. Goodlaw Student Travel Fellowship
Joanna Toner, University of California Berkeley
Robert D. Newcomb Student Travel Fellowship Award for Leadership
Alison Jensen, University of California Berkeley
Michael G. Harris Student Travel Fellowship Award for Leadership
Bibin Cherian, Northeastern State University of Oklahoma
AAO Student Travel Fellowships
Deborah Awisi-Gyua, Indiana University
Lacey Haines, University of Waterloo
Neeraj Singh, Indiana University
Sandra Wagner, Eberhard Karls University
Tiong Peng Yap, OD; University of New South Wales
Funded by an educational grant from Johnson & Johnson Vision
Nicole Adam, New England College of Optometry
Michelle Antonucci, University of California Berkeley
Akosua Asare, OD; Kwame Nkrumah University of Science and Technology
Khob Bhandari, University of Houston
Jaime Etterling, The Ohio State University
Raymond Farmer, State University of New York
Hannah Gregory, University of Houston
Brian Helmetag, Midwestern University Chicago College of Optometry
Chuan Hu, OD; University of Houston
Parthasarathi Kalaiselvan, University of New South Wales
WeiHau Law, University of Houston
Taylor Norris, The Ohio State University
Sudan Puri, University of Houston
Casey Ramirez Cortes, The Ohio State University
Noor Haziq Saliman, The University of Manchester
Divya Senthilkumar, SRM Institute of Science and Technology
Jennyffer Smith, OD; University of Houston
Emma Stewart-Bates, The Ohio State University
Kelsey Trast, University of Houston
Kent Uehara, State University of New York
Yen-Chiao Wang, MD; Indiana University
Recipients will be honored at the Student and Resident Awards Lunch from noon to 1 p.m. on October 24 in the Orange County Convention Center.
This week in optometry news: August 26, 2019
Alcon launches Precision1 contact lenses
Alcon has announced plans to launch the newest addition to its contact lens portfolio-Precision1 daily disposable, silicone hydrogel (SiHy) contact lenses-in the U.S.
Precision1 contact lenses are made with Alcon’s SmartSurface technology, a permanent, micro-thin, high-performance layer of moisture at the lens surface that helps support a stable tear film.
Alcon launched Precision1 contact lenses in Australia and New Zealand in March. The company expects to launch these lenses in additional markets around the globe throughout 2020 and 2021.
According to the company, Precision1 lenses are aimed at new contact lenses wearers and patients looking for lasting performance, strong visual performance, and strong comfort.
Precision1 is available in an 8.3 mm base curve with powers ranging from +8.00 D to -12.00 D. The lens is launching with the full parameter range.
Alcon is interested in expanding Precision1 to toric and multifocal designs in the future.

Top story: Remembering the life of Dr. John Keriotis
Mike Brown, OD, MHS-CL, FAAO, takes a look at the life of his longtime friend and colleague John Keriotis, OD, and the lasting memory he leaves behind in the optometric community.
Read it here!
CooperVision responds to FTC on proposed changes to Contact Lens Rule
CooperVision has submitted comments to the United States Federal Trade Commission’s (FTC) regarding its proposed changes to the Contact Lens Rule.
In its letter, CooperVision emphasizes five areas of consideration for the CDC:
• Patient ocular health and safety should be kept at the forefront of the FTC’s concerns.
• Eyecare professional expertise in selecting contact lenses for each patient is vital with no place for brand or prescription substitution-a view also held by the FTC.
• Providing prescriptions to patients is best practice. Education and enforcement-not increased administrative burdens applied equally across the profession-are key to increasing compliance.
• When consumers buy contact lenses from non-prescribing retailers, stronger FTC enforcement of the Contact Lens Rule’s provisions will ensure patient well-being.
• Technology should be leveraged to modernize the Contact Lens Rule, with electronic platforms playing a larger role in prescriber-seller-wearer communications, as well as the elimination of antiquated robocall verifications in favor of modern means like email.
Popular story: How to build a myopia control practice
Understand why myopia control is a burgeoning part of optometric practice, and learn new ways to starting to offer it in your own practice.
Find out more!
Sun Pharma, CMS enter agreement for generic product license
One of Sun Pharmaceutical’s wholly owned subsidiaries has granted an exclusive license to a subsidiary of China Medical System (CMS) Holdings Ltd. to develop and commercialize seven generic products in mainland China.
The collaboration with CMS now covers eight generic products. The total addressable market size for all eight products totals $1 billion in mainland China, according to recent IQVIA data.
The initial term of the agreement will be 20 years from the first commercial sale of the respective products in mainland China and may be extended for an additional three years, as per mutual agreement of the two parties.
Popular story: How to treat dry eye in the pediatric and young adult population
When ODs think about dry eye, they quickly associate classic demographics: Over-40 menopausal female patients taking a high number of medications or individuals with autoimmune disease. But are ODs overlooking a younger, seemingly healthy demographic?
Find out!
FDA approves new Paragon contact lens manufacturing facility
The U.S. Food and Drug Administration (FDA) has approved Paragon Vision Science’s newly-constructed contact lens manufacturing site in suburban Phoenix. Paragon is part of CooperVision Specialty EyeCare.
The Gilbert, Az. facility will produce ortho-k products such as Paragon CRT for use in the United States. The new site is more than double the size of the company’s current plant in Mesa, Az. The Mesa location will continue to fabricate lenses for global markets after U.S. production is transferred to Gilbert over the next several months.
Popular story: Understanding the basics of cataract surgery measurements
Technicians play an invaluable role in eye care. Apart from performing eye tests and measurements, the technician is also something of a detective, obtaining key elements of the patient history and learning how to ask additional questions when the pieces of the puzzle don’t quite fit.
Read on!
ASCRS Foundation announces 4th annual National Sight Week
The American Society of Cataract and Refractive Surgery (ASCRS) Foundation has announced its 4th annual National Sight Week for the week of October 20-26.
National Sight Week is a week-long celebration of volunteerism where members of the ASCRS Foundation’s Operation Sight Network are encouraged to contribute one or more charitable cataract surgeries in their own communities.
Operation Sight is the ASCRS Foundation’s U.S.-based charitable cataract surgery program. It involves a nationwide network of ASCRS volunteer surgeons and staff members who provide care to those unable to access or afford surgery on their own.
Since its launch in late 2014, Operation Sight has delivered over 3,530 free surgeries.
Foundation staff will match new and returning volunteers with eligible patients during National Sight Week.
For more information about Operation Sight and National Sight Week, visit their website.

Top story: Modernize contact lens wear in 2019
New advances in the contact lens sphere warrant specialty fits, additional coaching, and more cost to patients. But such technology can also change a patient’s quality of life for the better.
Learn how!
New World Medical launches new glaucoma drainage device
New World Medical, Inc. has launched the Ahmed ClearPath glaucoma drainage device.
Developed in partnership with glaucoma surgeons, the Ahmed ClearPath is the newest addition to the company’s Ahmed brand of drainage devices.
The Ahmed ClearPath is available in two sizes: 350 mm2 and 250 mm2. The two sizes target challenges in tube shunt surgery.
One feature of the Ahmed ClearPath implant isa flexible plate with a contour that conforms to the curvature of the eye. The suture fixation points are also positioned more anteriorly on the device compared to other valveless drainage devices.
The model 350 plate surface is positioned more posteriorly to avoid muscle attachment points, while the model 250 is designed to be a single quadrant implant that fits between the muscles. The device is available with an optional pre-threaded ripcord and a 23-GA needle.

Popular story: Controversies in pediatric refractive development
Pediatric refractive error development can be thought of in three stages: infancy, toddler, and childhood. Controversies in the measuring, development, and prescribing for refractive errors can create confusion for the practicing clinician.
Learn more!
Nidek launches Mirante Scanning Laser Ophthalmoscope
Nidek Co. has launched the Mirante Scanning Laser Ophthalmoscope.
Mirante is a multi-modal fundus imaging platform that combines high-definition scanning laser ophthalmoscopy (SLO) and optical coherence tomography (OCT) with ultra-wide field imaging.
The platform captures high-quality color images, fluorescein angiography (FA), indocyanine green angiography (ICG), fundus autofluorescence (FAF), retro mode images, OCT scan, and OCT-angiography (OCTA).
The optional wide-field adapter enables 163° ultra-wide field imaging with a single image capture.
Combined with 4096 x 4096 pixels imaging quality and ultra 4K HD, the Mirante captures a wider view of the retinal structure and vasculature. The new Flex Track algorithm corrects image distortion and enhances image averaging quality.
For color imaging, three red, green, and blue (RGB) detectors scan different depths of the retina with RGB wavelengths, producing color, and allowing adjustment of the histogram.
Dynamic blood flow using FA and ICG can also be recorded with the Mirante. The platform allows simultaneous acquisition of FA and ICG images.
The retro mode modality is a non-invasive technique for visualizing pathologies deeper than the retinal pigment epithelium and detecting pathologic changes in the choroid.
High-definition OCT images can be acquired for a maximum 16.5 x 12 mm area, allowing a wider and detailed assessment from the vitreous to choroid in a single shot. Mirante also offers options for AngioScan OCTA and an anterior segment OCT adapter.
This week in optometry news: August 12, 2019
Glaukos and Avedro announce definitive acquisition agreement
Glaukos Corporation and Avedro, Inc. announced that the companies have entered into a definitive merger agreement under which Glaukos will acquire Avedro in an all-stock transaction.
The transaction-subject to Avedro stockholder approval and other customary closing conditions and regulatory approvals-has been approved by the board of directors of both companies. It is expected to be completed in the fourth quarter of this year.
Once combined, the addition of Avedro’s 66-percent year-over-year revenue growth in the first half of 2019 is expected to generate revenue growth acceleration for Glaukos beginning in 2020, as well as potential revenue synergies beginning in 2021, according to the companies.
Upon closing, Glaukos shareholders are expected to own approximately 85 percent of the combined company, and Avedro shareholders are expected to own the remaining 15 percent.

Top story: New research on improving contact lens comfort for patients with dry eye
The latest Defocus Media podcast features Chandra Mickles, OD, MSc, FAAO, FSLS, as she discusses a recent study that has made a big impact on her prescribing philosophies when it comes to specialty contact lenses.
Read and listen!
Referendum effort on Arkansas eye surgery law rejected
Arkansas election officials rejected an attempt earlier this month to hold a referendum next year on a new state law that expands what procedures ODs can perform.
Supporters of the referendum fell short of the nearly 53,500 signatures from registered voters needed to put the issue on the November 2020 ballot, according to Arkansas Secretary of State John Thurston’s office. Thurston’s office said it determined the petitions submitted had 23,953 signatures.
The new law would allow ODs to perform including injections around the eye, removing lesions from the eyelids, and certain laser eye surgeries.
Supporters of the law say ODs are already trained to perform the procedures but are being forced to refer patients elsewhere.
Safe Surgery Arkansas, the group behind the referendum effort, says it is prepared to go to court to challenge the petitions’ rejection.
Safe Surgery says it spent more than $150,000 gathering signatures for the referendum bid and last week submitted more than 84,000 signatures. Supporters of the eyecare law had said many of the signatures were invalid because canvassers hadn’t filed necessary paperwork with the state.
Top story: 9 simple solutions to 9 complex cases
Sometimes contact lens fitters jump to “problem-solving” contact lenses with customized designs considering another-usually more simple-strategy can solve the challenge.
Learn more!
Poor eyesight and hearing loss costs billions, new UK report shows
A lack of accurate data is contributing to a £58 billion bill for vision and hearing loss in the UK, according to a report published calling on the government to support the first-ever national survey of the UK population’s sensory needs.
It is estimated that around 2 million people in the UK are affected by partial sight loss-which is expected to rise to 2.4 million by 2024. The number affected by hearing loss-also rising-is estimated at 11 million.
Researchers and charities have launched a campaign for the first-ever UK National Eye-health and Hearing Study (UKNEHS). The study includes 25,000 participants undergoing an eye and hearing examination as well as completing a standardized general questionnaire.
UKNEHS will also measure the detection and treatment coverage rate of major eye diseases and associated conditions, such as diabetes, in order to understand the effectiveness of current services.

Popular story: Scleral contact lenses help manage ocular surface disease
Many patients with corneal and ocular surface conditions also experience dry eye. Find out how scleral lenses can help to provide good vision, comfort, and ocular health.
Find out!
Abnormal hemoglobin levels linked to long-term dementia risk
High and low hemoglobin levels are associated with an increased risk of developing subsequent dementia, new research shows.
The latest results from the Rotterdam Study-a large, longitudinal population-based study-show individuals with anemia were 41 percent more likely to develop Alzheimer's disease (AD) and 34 percent more likely to develop any dementia type compared with individuals without anemia.
Investigators also found that those with high hemoglobin were also at greater risk of developing dementia.
The study was published online July 31 in Neurology.
Serum hemoglobin was measured in 12,305 participants (mean age, 64 years; 58 percent women). A total of 1520 people developed dementia during a mean follow-up of 12 years, 1194 of whom had AD.
The results showed a U-shaped association between hemoglobin levels and dementia (p=0.005), such that both low and high hemoglobin levels were associated with increased dementia risk.

Popular story: Offer more comfort to contact lens wearers
ODs have the opportunity to reduce the number of contact lens dropouts, but need to be proactive in the way they ask questions in the exam room and, ultimately, offer patients the highest-quality contact lens designs and optical properties available.
Find out how!
Novel drug candidate with marine origins may treat vision loss
Researchers from the University of Florida and Singapore are focused on a novel drug candidate with marine origins as a new method to prevent or treat vision loss.
UF College of Pharmacy researchers teamed with collaborators at the Singapore Eye Research Institute (SERI) and at A*STAR’s Institute of Molecular and Cell Biology (IMCB) in Singapore to see whether Apratoxin S4, a novel molecule based on marine cyanobacteria, could be an effective therapy in restricting abnormal blood vessel formation in the eye.
The research team discovered in laboratory testing that Apratoxin S4 inhibited the development of abnormal blood vessels in the eye but left normal blood vessel formation untouched.
Multiple methods of delivering the therapy were proven to be effective, including systemic drug delivery into the bloodstream or local drug delivery into the back of the eye. The researchers determined Apratoxin S4 can be a therapy on its own or work in combination with vascular endothelial growth factor (VEGF)-inhibiting drugs to stop the growth of abnormal blood vessels.
The study was published in the Investigative Ophthalmology & Visual Science in July.

Top-read story: How to diagnose a swollen optic nerve
There is not much more intimidating for an OD during a normal clinic day than for a patient to come in complaining of vision loss and the dilated fundus exams reveals a swollen optic nerve'(s). Find out how to work through the differential diagnosis.
Find out here!
Deep-learning AI may help curb smokers’ addiction
Newly-published research seeks to determine if a deep-learning approach can identify environments and environmental features associated with smoking.
In the cross-sectional study of 4,902 images of daily environments taken by 169 smokers, a deep-learning classifier was trained to identify environments associated with smoking.
The images were used to develop a probabilistic classifier to predict the smoking or non-smoking location and connect objects and settings in daily environments to established smoking patterns, according to the study.
Results indicated that specific objects and settings were associated with smoking, and significant correlation was found in participant-reported cravings after unfamiliar environments were viewed, researchers reported.
The findings suggest that a deep-learning approach can identify environments associated with smoking, predict the probability that an image of daily life represents a smoking environment, and can potentially trigger environment-based interventions.
This week in optometry news: August 5, 2019
EssilorLuxottica confirms GrandVision acquisition
EssilorLuxottica has announced an agreement with Hal Optical Investments (HAL) for the sale of HAL’s 76.72 percent ownership interest in Grand Vision.
Under the agreement, Essilor will purchase HAL for £28 a share-which will increase by 1.5 percent, or £28.42, if the acquisition is not closed within 12 months, the company says.
The transaction is expected to close within 12 to 24 months, according to the company. Through the acquisition, EssilorLuxottica will be adding more than 7,200 stores globally, 37,000 employees, and an annual revenue of $3.7 billion euros.

Popular story: New research on improving contact lens comfort for patients with dry eye
The latest Defocus Media podcast features Chandra Mickles, OD, MSc, FAAO, FSLS, as she discusses a recent study that has made a big impact on her prescribing philosophies when it comes to specialty contact lenses.
Read and listen!
Wills Eye, Bascom Palmer Institute take top honors in annual ‘Best Hospitals’ rankings

Wills Eye Hospital and Bascom Palmer Eye Institute of the University of Miami Health System have taken top ranking in the 2019-2020 U.S News & World Report “Best Hospitals” rankings.
The annual survey of “Best Hospitals” is determined by a survey of board-certified physicians throughout the U.S. Wills Eye was voted a top center of excellence, while Bascom Palmer took the top ranking for the 18th time since the publication began surveying U.S. physicians 30 years ago.
Bascom Palmer is also ranked as the No. 1 overall ophthalmology program, the best in clinical care, and the best ophthalmic residency program in the U.S. by Ophthalmology Times.
The published rankings can be viewed at http://health.usnews.com/best-hospitals/rankings.

Popular story: 3 mental health conditions to watch for in patients
About 10 percent of patients may have an undiagnosed or untreated mental health condition. ODs can make a difference in a patient’s life, possibly even saving it.
Find out how.
More Gen Z employees enrolling in vision benefits, Transition Optical survey finds
While vision benefit enrollment remains significantly higher among older employees, new research from Transitions Optical reveals that the trend may be changing.
Today, Gen Z employees are more likely to enroll in and utilize their company’s vision benefits than they were just one year ago.
According to the 10th annual Transitions Optical Employee Perceptions of Vision Benefits survey, six in 10 Gen Z employees (ages 18 to 24) are enrolled in a vision plan-compared to just half of Gen Z employees surveyed in 2018.
Additionally, 44 percent of Gen Z employees say that whether a company offers vision benefits has been an important factor in their decision to accept a job-reinforcing the value of talking up vision benefits when it comes to employee attraction and retention.
The survey also found that today’s Gen Z employees are not only more likely to be enrolled in vision benefits, but they are also more likely to use them.
Two-thirds (65 percent) are likely to visit an eyecare professional within the next 12 months, compared to around half (56 percent) in 2018.
Gen Z employees are just as likely to visit an eyecare professional as they are a primary-care physician.
When it comes to benefits enrollment overall for Gen Z employees (59 percent), vision is not far behind dental insurance (61 percent) or even medical insurance (71 percent). Gen Z employees are also significantly more likely to enroll in vision benefits than they are life insurance (41percent) or a 401k plan (38 percent).
According to the survey, eight in 10 Gen Z employees say premium lens options are important to them when selecting eyewear-with three out of four saying they would be more likely to enroll in or keep using a vision plan if it covered eyewear options like Transitions lenses.

Top story: 3 updates to treating Sjögren’s syndrome and dry eye
Take a look at the latest and most important updates in the treatment for Sjögren’s syndrome and dry eyes, such as new guidelines, a potential new medication, and taking another look at the Sjögren’s biomarker test.
Read more!
ABB Optical Group accepting nominations for ABB Cares Program
In the past five years, ABB Optical Group’s ABB Cares community grants program has awarded non-profit organizations across the country with more than $65,000 in grants. Applications for the sixth annual ABB Cares program are being accepted now through August 31.

This year the company will award one ABB Cares platinum grant of $5,000, two gold grants of $2,500 each, and four silver grants of $1,000 to charities nominated by professionals in the eyecare industry. Organizations do not need to focus on eye health to qualify for a grant.
To be considered as a grant recipient, all candidates must comply with the following:
• The organization must be a 501(c)(3) organization and provide proof of that status.
• The organization's local office must be within 30 miles of the nominating practice's primary location.
• The organization must be located in the United States.
• All applications must be submitted in English.
• Applications must be completed and submitted online.
• The deadline to apply is 11:59 p.m. ET on August 31.
For more information or to submit an application, visit https://abboptical.com/ABBCares.
This week in optometry news: July 29, 2019
August recognized as Children’s Eye Health Month
For the second year in a row, Prevent Blindness and the National Optometric Association (NOA) are teaming up to declare August as Children’s Eye Health and Safety Awareness Month.
The National Center for Children’s Vision and Eye Health at Prevent Blindness is offering the newly revised “
Guide to Vision Health for Your Newborn, Infant, and Toddler” in celebration of its 10th anniversary.
This comprehensive resource offers information on topics such as common milestones for visual development, how to help a baby’s vision to develop, and warning signs of possible vision problems.
A child may be at higher risk of developing a vision problem if he:
• Was born prematurely (less than 32 weeks completed gestation.)
• Has a family history of vision disorders such as childhood cataract, misaligned eyes, eye tumors, or wore glasses before first grade.
• Has had an eye injury (problems resulting from childhood eye injuries may develop much later in life.)
• Has been diagnosed with a problem that could affect his physical, mental, and/or, emotional development.

Top story: New research on improving contact lens comfort for patients with dry eye
The latest Defocus Media podcast features Chandra Mickles, OD, MSc, FAAO, FSLS, as she discusses a recent study that has made a big impact on her prescribing philosophies when it comes to specialty contact lenses.
Read more!
Transitions Optical launches Facebook virtual try-on experience

Transitions Optical has partnered with Facebook to create an augmented reality try-on experience using the Facebook camera.
The new Facebook virtual try-on from Transitions Optical is a first-of-its-kind social media experience allowing users to see themselves in all 13 Transitions lens colors-including Transitions Signature lenses style colors and Transitions XTRActive style mirrors. Users can browse between indoor and outdoor lens settings.
Users can activate the Transitions Facebook Try-on by clicking a link located on the Transitions Facebook Page, or by scanning the QR code with their Facebook app.
Eyecare professionals can visit TransitionsPRO.com/FacebookTryOn to download resources.

New blog: Examine evaporative dry eye disease exposure in your patients
Know how to recognize and address the underlying cause(s) of evaporative DED influences in your patients before more serious and chronic problems occur.
Find out!
Frequent sleeping pill use linked to increased risk for dementia
New research reported at the Alzheimer’s Association International Conference (AAIC) 2019 in Los Angeles evaluated drug and non-drug treatments to improve sleep patterns in persons with Alzheimer’s disease or other dementias.
More frequent use of sleep medications may be associated with higher risk of dementia, especially in older white adults compared to older black adults who experienced reduced risk. Whether the change in risk is due to the medications or sleep problems is not yet known.
Men over 65 years of age who used sleep medications had an increased risk of developing Alzheimer’s dementia, as were some women. However, women who used sleep medications but did not have interrupted sleep had a reduced risk for the disease
An observational study of 3,656 adults 65 and older (57.8 percent female) from the Cache County Study on Memory and Aging (CCSMA) evaluated if use of sleep medications was associated with an increased risk of developing Alzheimer’s, and whether risk differed between men and women.
The study found that men who reported use of sleep medication had a 3.6 times increased risk of developing Alzheimer’s disease compared to those who did not use sleep medications.
In women, the risk varied by whether or not they experienced sleep disturbances. Women who used sleep medications but did not report sleep disturbances were at nearly four times greater risk for developing Alzheimer’s.
Women who said they had sleep disturbances and used sleep medications were at a 35.2 percent reduced risk of developing Alzheimer’s.

Popular story: Explore the relationship between dry eye and sleep
Chronic sleep problems affect 70 million adults in the U.S. Find out why a restless night of sleep may be to blame for the development of DED in your patients.
Find out!
Innovega earns IRB approval for on-eye testing of iOptik contact lens
Innovega Inc. has received institutional review board (IRB) approval of a protocol for single exposure of its iOptik contact lens and prototype display eyewear simultaneously for up to six hours.
This will allow augmented reality subject experts to report their observations when viewing media from Innovega's eMacula near-eye display eyewear.
The company expects to receive two additional IRB approvals that will allow for further feasibility clinical studies at The Ohio State University. Results are anticipated to be available by early fourth quarter of this year, according to the company.
Each of these studies will provide data for the design of the subsequent pivotal Phase III studies.
The first will be a 510(k) study involving just the contact lens material, while the second will be for the iOptik contact lens for viewing eMacula near-eye display eyewear.
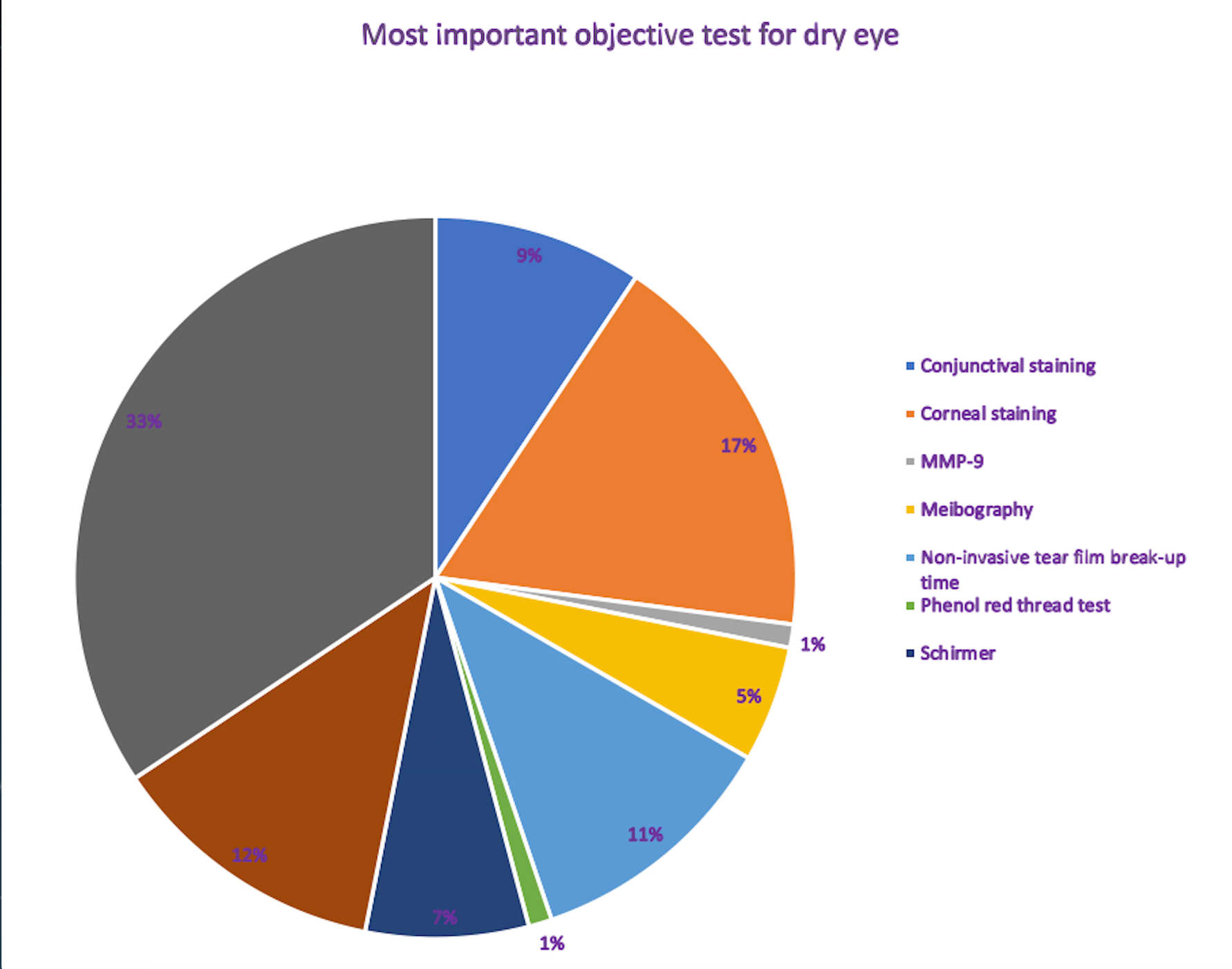
Poll results: The most important objective dry eye test is TBUT with fluorescein, ODs say
Find out what your peers says is the most important objective dry eye tests from eight choices.
Find out!
Total ECP and Visionary Eye Partners merge to form Keplr Vision
Imperial Capital-backed Total ECP and Visionary Eye Partners have merged to form Keplr Version.
Keplr is a business support services company built to invest in medically focused optometry practices, as well as provide lens technology and patient experience.
Along with Nick Williams as chief executive officer (CEO), Keplr is led by Chief Medical Officer (CMO) Ben Gaddie, OD, FAAO, and Chief Operating Officer (COO) Adam Rosengren.
Keplr's medical leadership team includes:
• Alan Glazier, OD, FAAO
• Neil Gailmard, OD, MBA, FAAO
• Paul Karpecki, OD, FAAO
• Eric Schmidt, PhD
Jay Binkowitz serves as the company's executive vice president of professional relations and works to develop new investment opportunities for Keplr.

Top story: Offer more comfort to contact lens wearers
Find out how to address the ocular surface with strategies backed by research
Find out!
DrChrono teams with DeepScribe to automate medical note taking in EHR via AI tech x
DrChrono Inc announced a partnership for medical professionals to utilize artificial intelligence (AI) in automating medical note-taking from physician appointments and integrate it into their electronic health record (EHR).

DeepScribe uses advanced deep-learning technology to generate accurate, compliant, and secure simple object access protocol (SOAP) notes directly within a practice’s EHR, according to the company.
Practices using DeepScribe have reported saving an average of 2 hours and 45 minutes per day on documentation, with providers seeing an average of four to five additional patients, and in some cases, up to 10 additional patients, per week, according to the company.
Practices using DrChrono’s EHR platform can use the DeepScribe app and device to automate the note-taking process and have those notes available in the patient record.
Providers can record their patient exams, with patients' permission, by opening their DeepScribe app within DrChrono’s EHR platform.
Similar to the Apple HomePod, Amazon Echo, or Google Home, the DeepScribe app sits in the exam room and listens to the conversation and prepares notes. The notes show up within the DrChrono EHR within an hour, and providers are able to review and sign off on the notes.
This week in optometry news: July 22, 2019
FDA approves Allergan’s NDA for bimatoprost SR for open-angle glaucoma, ocular hypertension
Allergan plc announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s New Drug Application (NDA) for bimatoprost sustained-release (SR).
If approved, Bimatoprost SR would be the first-in-class sustained-release, biodegradable implant for the reduction of intraocular pressure (IOP) in patients with primary open-angle glaucoma or ocular hypertension, according to the company.
The FDA is expected to take action on the NDA by the end of the first half of 2020.
In the two Phase 3 A Robust TNBC Evaluation framework to Improve Survival (ARTEMIS) studies, bimatoprost SR reduced IOP by 30 percent over the 12-week primary efficacy period, meeting the predefined criteria for non-inferiority to the study comparator, according to the company.
The ARTEMIS studies evaluated 1,122 subjects on the efficacy and safety of bimatoprost SR versus timolol in patients. After three treatments with bimatoprost SR, >80 percent of patients remained treatment free and did not need additional treatment to maintain IOP control for at least 12 months.

Top story: Offer more comfort to contact lens wearers
Find out how to address the ocular surface with strategies backed by research
Find out!
EssilorLuxottica confirms discussions to purchase ownership interest in GrandVision
EssilorLuxottica has confirmed it is having discussions to purchase the 76.72 percent ownership interest in GrandVision N.V. of HAL Holding N.V.
No agreement has been reached, and no assurance can be provided that these discussions will lead to such an agreement, according to the company.
Completion of the transaction would be subject to customary conditions precedent, including approval by regulatory authorities in various jurisdictions.
The purchase price per share indicatively discussed among the parties to date is 28.00 per share. The terms of the potential transaction, including price, will be subject to continued evaluation and discussion during the coming weeks as well as further review by the parties’ boards of directors.

Top story: Scleral contact lenses help manage ocular surface disease
Many patients with corneal and ocular surface conditions also experience dry eye. Find out how scleral lenses can help to provide good vision, comfort, and ocular health.
Find out!
UAVenture Capital Fund invests in automatic eyeglass prescription technology
UAVenture Capital Fund (UAVC) announced its seventh portfolio investment and the second investment from Fund II.
The Fund has invested in iCrx, Inc., a Wyant College of Optical Sciences technology that will introduce a compact automatic phoropter utilizing laser technology designed to instantly determine a required eyewear prescription.
The iCrx invention is a hand-held device that can produce an objective and accurate eyeglass prescription in approximately 20 seconds without any subjective input from the viewer, according to the company.
The invested funds from UAVC will be utilized to further develop and miniaturize the technology into a commercial product to be used in kiosks and/or in rapid screening of large patient populations, particularly in developing countries lacking sufficient facilities and specialist doctors.
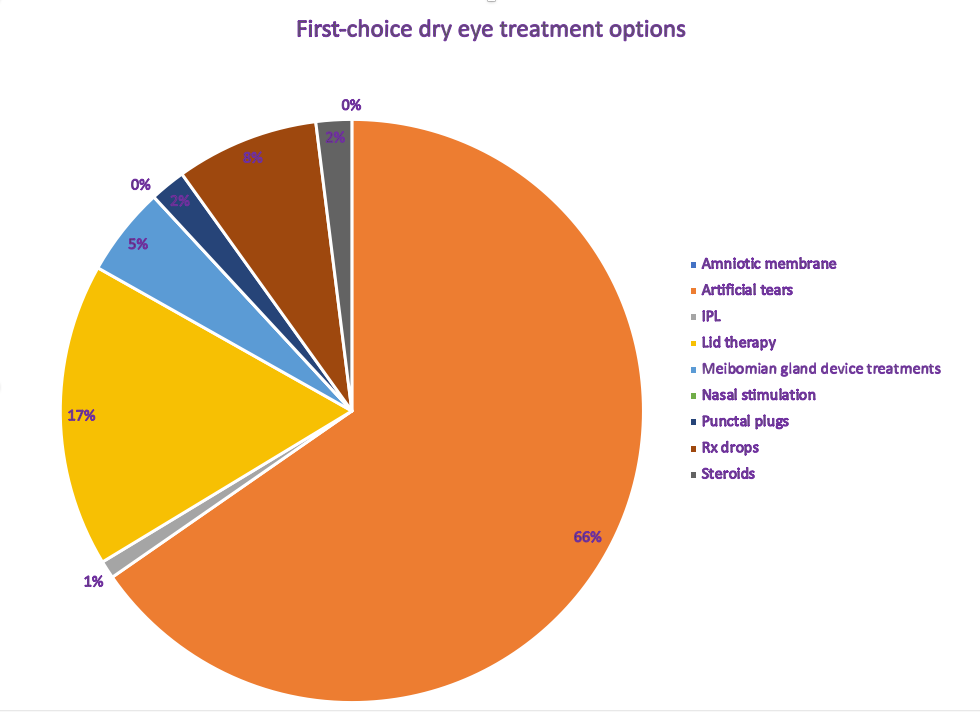
New story: ODs reach for artificial tears first
See how dry eye first-choice treatment options stack up in our poll results.
Read them here!
Hearing, vision loss raises risk for dementia in older adults
Two studies reported at the Alzheimer’s Association International Conference (AAIC) 2019 found that experiencing multiple sensory impairments, such as vision and hearing problems, are associated with an increased risk of developing dementia in older adults.
Research from the University of Washington School of Public Health showed that impairment of vision or hearing increases the risk of developing dementia, and that impairment in both senses further increases those odds.
Meanwhile, researchers at the University of California, San Francisco, studied the combined effects of loss of smell, touch, vision, and hearing. They found that even mild impairments in multiple senses were associated with an increased risk of dementia and cognitive decline.
Having either visual or hearing impairment increased the risk of developing dementia by 11 percent and Alzheimer’s disease by 10 percent.
Having both visual and hearing impairment raised the risk of developing dementia by 86 percent and Alzheimer’s disease by 112 percent.

Popular story: Explore the relationship between dry eye and sleep
Chronic sleep problems affect 70 million adults in the U.S. Find out why a restless night of sleep may be to blame for the development of DED in your patients.
Find out!
Clerio Vision acquires manufacturer of Extreme H20 contact lenses
Clerio Vision, Inc. has acquired Hydrogel Vision Corporation (HVC).
HVC is best known for its Extreme H20product line. HVC offers contact lenses in multiple diameters, with smaller lenses to more comfortably fit those with smaller corneas or narrower eyelids, and larger lenses for those with larger corneas or for improved sports performance.
HVC contact lenses are made from a proprietary ultra-hydrating material that retains up to 99 percent of its moisture, according to the company. HVC distributes the only disposable opaque color contact lenses for those with astigmatism.
Clerio was founded in 2014 to commercialize femtosecond laser research at the University of Rochester. Clerio's multifocal contact lens product is currently in clinical development and is expected to be on the market in the next 18 months.

New blog: Why dry eye?
One OD explains why she took on the challenge of creating a dry eye center, and how best to treat this patient base.
Learn more!
CORE director tops global contact lens list
Expertscape has recognized multiple scientists and researchers at the Centre for Ocular Research & Education (CORE) as among the world’s most respected contact lens authorities.
Lyndon Jones, PhD, DSc, FCOptom, FAAO, director of CORE, was ranked as the top global expert. The University of Waterloo placed second among institutions.
The biomedical-focused website objectively ranks people and institutions by their expertise in more than 27,000 topics, based on searches of PubMed-logged articles spanning the past decade.
Expertscape allows healthcare professionals and consumers to find the best institutions, leading experts, and latest publications on a range of medical subjects.
The entire contact lens ranking can be accessed here.
Four current CORE team members are ranked among the top 60 contact lens experts:
1. Lyndon Jones, PhD, DSc, FCOptom, FAAO, CORE director, professor
23. Desmond Fonn, MOptom, FAAO distinguished professor emeritus and founding director
48. Doerte Luensmann, PhD, Dipl Ing (AO), FAAO, senior clinical scientist
58 Chau-Minh Phan, MSc, PhD, postdoctoral fellow
Two faculty members at the School of Optometry & Vision Science at the University of Waterloo are also among the top 60:
25. Luigina Sorbara, OD, MSc, FAAO, professor
45. Trefford Simpson, MSc, PhD, DipOptom, professor
The list also includes four former CORE staff members who have transitioned to roles across the ophthalmic spectrum.
CORE is represented on Expertscape’s contact lens solutions global experts’ list with four current and former staff members included in the top 20. Dr. Jones is ranked number two.

New blog: Examine evaporative dry eye disease exposure in your patients
Know how to recognize and address the underlying cause(s) of evaporative DED influences in your patients before more serious and chronic problems occur.
Find out!Nidek launches LPM, low power mode for YLC-500 Vixi
Nidek Co. Ltd has launched LPM optional software, a low-power mode for the YLC-500 Vixi laser photocoagulator.
LPM provides a therapeutic effect to the retina by decreasing laser power during photocoagulation, according to the company. The standard laser power is decreased by a specified ratio during treatment, achieving minimally invasive photocoagulation to the macular region.
Features of this laser include an auto forward function and arcade grid scan pattern for laser delivery. Because no visible signs of photocoagulation are present during LPM treatment, these features are included to provide feedback during laser emission.
The auto forward function automatically advances the scan pattern to the next position. The arcade grid scan pattern prevents treatment in a central circular area within the grid. A software upgrade is required for incorporating LPM into the YLC-500 Vixi.
Top story: Why ODs shouldn't stop short with patient care
When focusing on managing a contact lens patient whose complaints have quieted, ODs may be tempted to stop shy of providing a thorough examination. One case shows why complete follow-through is crucial for proper treatment.
Learn more!
Costa’s Waterwoman frame takes best of category eyewear title at ICAST 2019
Costa’s Waterwoman frame won the title of Best of Category: Eyewear during the International Convention of Allied Sportfishing Trades (ICAST) 2019 New Product Showcase Awards in Orlando earlier this month.
This marks Costa’s first female style that has won an ICAST award, and the 11th ICAST award Costa products have won for various new sunglass styles and lens technologies.
Designed for women with active, water-based lifestyles, the Waterwoman is a medium frame with angled temples. The thick-rimmed frame features hand-painted sketches on the bio-resin nylon.
Now available, the sunglass style features integral hinges with a locking CAM system, slip-resistant Hydroliteover-injected temple pads and inset nose pads. Available colors include matte shadow tortoise, shiny palm tortoise, shiny wahoo, and shiny blond crystal.
This week in optometry news: July 15, 2019
Essilor celebrates launch of Transitions Signature Gen 9, Essilor Next Gen Offer
Essilor of America has launched the Essilor Next Gen Offer, a consumer promotion designed for eyecare professionals to recommend the new Transitions Signature Gen 8lenses to more patients.
From July 10 through December 31, when patients purchase Transitions Signature Gen 8 lenses with select Essilor lenses, they can receive a free bonus pair of clear, qualifying lenses with frame purchase.
When patients purchase Transitions Signature Gen 8 lenses with any Crizal, they can choose among Varilux X series, Eyezen, or Essilor single vision lenses to qualify for a free pair of lenses with the offer.
For their free clear pair, patients can select any Crizal on any Varilux, Eyezen, or Essilor single vision lenses.
Essilor will launch a consumer advertising campaign consisting of national TV, connected TV, digital advertising-including video and display ads-in-store advertising, and geo-targeted and smart search ads.

Popular story: Explore the relationship between dry eye and sleep
Chronic sleep problems affect 70 million adults in the U.S. Find out why a restless night of sleep may be to blame for the development of DED in your patients.
Find out!
Children’s concussion symptoms may persist for a year
A year after a concussion, up to one-third of kids still have symptoms such as headache and irritability that may affect school performance, a new study published online in Pediatrics finds.
A team lead by Linda Ewing-Cobbs, a professor of pediatrics at the University of Texas Health Science Center Medical School in Houston, found that as many as 31 percent of children still had symptoms-such as inattention or fatigue-12 months after their head injuries.
Girls with mood problems beforehand and kids from poor or troubled families seem the most vulnerable, researchers say.
Researchers looked at nearly 350 children, ages 4 to 15, who suffered either a concussion or an orthopedic injury. Parents completed surveys asking about their kids before the injury and general information about their home lives.
The researchers then used a ratings scale to evaluate post-concussion recovery.
Although girls and boys had similar pre-concussion characteristics, girls had significantly more persistent symptoms than boys. They also had twice the odds of symptoms lasting one year after injury, findings showed.
Family dynamics were also an important factor in children's recovery, study authors noted.

Top story: Scleral contact lenses help manage ocular surface disease
Many patients with corneal and ocular surface conditions also experience dry eye. Find out how scleral lenses can help to provide good vision, comfort, and ocular health.
Find out!
Biomarkers in blood may help predict recovery time post-concussions
A study of high school and college football players suggests that biomarkers in the blood may have potential use in identifying which players are more likely to need a longer recovery time after concussion, according to a study published in the online issue of Neurology.
The study involved 41 high school and college football players who experienced a concussion during the season. None of the players lost consciousness with their concussions. The players were matched with 43 football players of the same level, age and position who did not have a concussion during that season.
All participants had blood tests at the beginning of the season. Those who had concussions had blood tests within six hours after the injury, then again 24 to 48 hours later and also eight, 15, and 45 days later. Those who did not have concussions had tests at similar times for comparison.
The tests looked at levels of seven biomarkers for inflammation that have been related to more severe brain injury. Of the seven biomarkers, two were elevated for those with concussion at six hours after the injury compared to the athletes with no concussion. The biomarkers interleukin 6 and interleukin 1 receptor antagonist were both elevated at six hours after concussion.
For interleukin 6, levels at the beginning of the study were 0.44 pg/mL for those who later had concussions and 0.40 pg/mL for those who did not have concussions. At six hours after the injury, those with concussions had levels of 1.01 pg/mL, compared to levels of 0.39 at a similar time for those without concussions.
Athletes with higher levels of interleukin 6 six hours after the injury were also more likely to take longer to recover from their symptoms. Overall, the athletes with concussions had symptoms for an average of 8.9 days. Eight of the 17 athletes with concussion and high interleukin 6 levels at six hours after injury, compared to their levels at the beginning of the season, still had concussion symptoms eight days after the injury.
Limitations of the study included that the number of athletes with concussion may not be large enough to adequately determine the accuracy of inflammatory markers as biomarkers. and that the study included only male high school and college athletes-so the results may not apply to female or youth athletes.

Top story: 9 simple solutions to 9 complex cases
Sometimes contact lens fitters jump to “problem-solving” contact lenses with customized designs considering another-usually more simple-strategy can solve the challenge.
Read more!
GenSight Biologics completes enrollment early for Phase II trial against LHON
GenSight Biologics announced that enrollment in Efficacy & Safety Study of Bilateral IVT Injection of GS010 in LHON Subjects Due to the ND4 Mutation for up to 1 Year (REFLECT), a Phase III clinical trial of GS010 for the treatment of Leber’s hereditary optic neuropathy (LHON), was successfully completed ahead of schedule.
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the efficacy and safety of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation. Enrolling the target number of 90 subjects was originally anticipated to be completed in September 2019; instead, the 98th subject enrolled in the trial was treated on July 2.
The trial enrolled subjects with vision loss up to one year in duration and is underway across multiple centers in the U.S., Europe, and Taiwan. In the active arm, GS010 was administered as a single intravitreal injection to both eyes of each subject. In the placebo arm, GS010 was administered as a single intravitreal injection to the first affected eye, while the fellow eye received a placebo injection.
The primary endpoint for the REFLECT trial is the best-corrected visual acuity (BCVA) change from baseline reported in LogMAR at 52 weeks post-treatment in the second affected/not yet affected eye.
Secondary efficacy endpoints include: BCVA reported in LogMAR at two years post-treatment in the second affected/not yet affected eye compared to both placebo and the first affected eye receiving GS010, OCT, contrast sensitivity, and quality of life.
The first subject was treated in March 2018; topline Week 52 results are expected to be available in the third quarter of 2020.
This week in optometry news: July 10, 2019
Altaire issues voluntary recall on 23 eyecare products
Altaire Pharmaceuticals has voluntarily recalled 150 lots of 23 ophthalmic products due to the potential for nonsterility. The eye drops and ointments were manufactured and labeled exclusively for Walgreens, Walmart, and Perrigo, and sold over the counter or by prescription in those retail stores.
The recall is a precautionary response to quality assurance concerns at the manufacturing facility, according to the company. No injuries have been reported, and all recalled products have passed sterility testing.
The company has advised stores to return all affected units, and for patients to discontinue use of the following products immediately:
Recalled Walgreens products
• Lubricant Eye Drops Moisturizing, 15 mL
1 lot; NDC: 0363-0185-13
• Lubricant Eye Drops Moisturizing Twin Pack, 2 x 15 mL
1 lot; NDC: 0363-0185-49
• Sodium Chloride Ophthalmic Ointment, 5% hypertonicity eye ointment, 3.5 gm
1 lot; NDC: 0363-7500-50
• Sodium Chloride Ophthalmic Solution, 5% hypertonicity eye drops, 15 mL
2 lots; NDC: 0363-0193-13
• Lubricant Eye Ointment PF Soothing, 3.5 gm
1 lot; NDC: 0363-0191-50
Recalled Walmart products
• Equate Restore Tears Lubricant Eye Drops Twin Pack, 2 x 15 mL
4 lots; NDC: 49035-189-49
• Equate Eye Allergy Relief Drops, 15 mL
15 lots; NDC: 49035-887-13
• Equate Sterile Lubricant Stye Ointment, 3.5 gm
8 lots; NDC: 49035-875-50
• Equate Comfort Gel Lubricant Eye Gel Twin Pack, 2 x 15 mL
6 lots; NDC: 49035-197-49
• Equate Restore PM Nighttime Lubricant Eye Ointment, 3.5 gm
18 lots; NDC: 49035-191-50
• Equate Night & Day Restore Tears Lubricant Eye Pack, 3.5g and 15mL
4 lots; NDC: 49035-883-59
• Equate Support Advanced Twin Pack, 2 x 15 mL
2 lots; NDC: 49035-885-49
• Equate Support Advanced Lubricating Eye Drops Dose Preservative Free, 25 count (0.6 mL fill)
7 lots; NDC: 49035-882-54
• Equate Support Advanced Lubricant Gel Drops Multi Dose Preservative Free, 7.5 mL
4 lots; NDC: 49035-882-52
• Equate Support Moisture Lubricant Eye Drops, 10 mL
5 lots; NDC: 49035-145-10
• Support Harmony Lubricant Eye Drops, 10 mL
1 lot; NDC: 49035-145-10
Recalled Perrigo products
• Neomycin and Polymixin B and Bacitracin Zinc Ophthalmic Ointment, 3.5 gm
2 lots; NDC: 0574-4250-35
• Neo-Poly Dex (neomycin, polymyxin B, and dexamethasone) Ophthalmic Ointment, 3.5 gm
28 lots; NDC: 0574-4160-35
• Neo-Polycin HC (neomycin, polymyxin B, bacitracin zinc, and hydrocortisone acetate) Ophthalmic Ointment, 3.5 gm
4 lots; NDC: 0574-4144-35
• Polycin (polymixin B and bacitracin zinc) Ophthalmic Ointment, 3.5 gm
12 lots; NDC: 0574-4021-35
• Bacitracin Ophthalmic Ointment, 3.5 gm
11 lots; NDC: 0574-4022-35
• Sulfacetamide Sodium Ophthalmic Ointment, 3.5 gm
2 lots; NDC: 0574-4190-35
• Puralube Ophthalmic Ointment, 1-3.5 gm
11 lots; NDC 0574-4025-35 and 0574-4025-20
Chief Optometric Editor Ben Casella, OD, FAAO, shares the quick-and-easy fix he found to treat his son’s hypersensitivity to their family cat, and why the experience has led him to re-examine his approach to treating patients with similar conditions.
Read more!AOA releases evidence-based clinical practice guideline for peer, public review
The American Optometric Association (AOA) has released the second edition of its evidence-based clinical practice guideline, Evidence-Based Guideline on Eye Care of the Patient with Diabetes Mellitus, for an online 30-day public review period, until July 30.
The guideline describes appropriate examination procedures for evaluation of the eye health and vision status of patients with diabetes mellitus to ensure appropriate care.
This AOA guideline meets the Institute of Medicine (IOM)-now the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine-standards as outlined in its report, Clinical Practice Guidelines We Can Trust.
Review of the second edition of the practice guideline provides the AOA EBO Committee with necessary feedback on how to reach multiple stakeholders with personal or professional interest in this guideline, according to Diane Adamczyk, OD, chair of the AOA Evidence-Based Optometry (EBO) Committee, which is responsible for compiling the evidence-based clinical practice guidelines.
The finished guideline will be available later in 2019.
Popular story: Troubleshoot contact lens discomfort and prevent complications
Ocular surface disease can be further complicated by contact lens wear. Keep an open mind to the possible underlying conditions, even with asymptomatic patients.
Learn more!
Novartis closes deal on Xiidra
Novartis announced that it has completed its acquisition of Xiidra (lifitegrast ophthalmic solution) 5%.
Xiidra is a prescription eye drop solution designed to treat the signs and symptoms of dry eye disease. It is dosed twice per day, approximately 12 hours apart, in each eye. Xiidra is approved to treat signs and symptoms of dry eye disease (DED) in multiple markets, including the U.S., Canada, and Australia. It is under regulatory review in a number of additional markets.

Top-read story: How to diagnose a swollen optic nerve
There is not much more intimidating for an OD during a normal clinic day than for a patient to come in complaining of vision loss and the dilated fundus exams reveals a swollen optic nerve(s). Find out how to work through the differential diagnosis.
Find out here!
Bausch + Lomb launches Ocuvite Eye Performance vitamins
Bausch + Lomb announced the U.S. launch of Ocuvite Eye Performance, an eye vitamin supplement formulated with seven vital nutrients to strengthen the macula, according to the company.
The Ocuvite Eye Performance nutrient formula contains high levels of seven vital nutrients including lutein and zeaxanthin-two carotenoid pigments naturally found in the eye, omega-3’s-to help support healthy tears and a healthy retina, zinc to help regulate immune function in the eye, vitamins C and E, and D.

Popular story: POLL: What treatment option do you choose when managing dry eye disease patients?
ODs have many choices when treating their dry eye patients. What’s your first choice?
Weigh in on our poll!
Alcon introduces Air Optix plus HydraGlyde for astigmatism
Alcon launched Air Optix plus HydraGlyde for astigmatism contact lenses in the U.S. The newest addition to Alcon’s contact lens portfolio offers a combination of technologies supporting long-lasting lens surface moisture and comfort to provide astigmatic patients a consistently stable lens-wearing experience, according to the company.
The new contact lenses include a blend of technologies:
• Proprietary HydraGlyde Moisture Matrix for long-lasting lens surface moisture
• Precision Balance 8|4 lens design to hold the lens in place with every blink
• SmartShield Technology that helps guard against irritating deposit build-up

Top story: Blog: 8 common cash flow pitfalls to avoid: Part I
Only 40 percent of all businesses survive beyond the first six years. Common cashflow pitfalls can occur in any business, including OD practices. Avoiding them can significantly stabilize, strengthen, and even increase the value of a business.
Learn how!
RNA therapy has potential to restore vision in patients with rare blindness disorder
ProQR Therapeutics announced an upcoming presentation at the 11th Annual Usher Connections Conference.

A presentation will be delivered by Aniz Girach, MD, chief medical officer of ProQR, on QR-421a for Usher syndrome during the USH2019 Annual Usher Connections Conference in Philadelphia on July 13.
To date, there are no treatments approved or products in clinical development that treat the vision loss associated with Usher syndrome type 2, one of the most common forms of Usher syndrome that can be caused by mutations in the USH2A gene.
QR-421a is a first-in-class investigational RNA-based oligonucleotide designed to address the underlying cause of vision loss in Usher syndrome type 2 and non-syndromic retinitis pigmentosa (RP) due to mutations in exon 13 of the USH2A gene, according to the company.
QR-421a is designed to restore functional Usher in protein by using an exon skipping approach with the aim to stop or reverse vision loss in patients.
The RNA therapy is intended to be administered through intravitreal injections in the eye. It has been granted orphan drug designation in the U.S. and the European Union, after having received fast-track designation from the Food and Drug Administration (FDA).

Top story: Explore the relationship between dry eye and sleep
Chronic sleep problems affect 70 million adults in the U.S. Find out why a restless night of sleep may be to blame for the development of DED in your patients.
Find out!
National Stem Cell Foundation launches first 3D human model of Parkinson’s disease, Progressive MS to ISS
The National Stem Cell Foundation (NSCF) announced that research teams from the New York Stem Cell Foundation (NYSCF) Research Institute, Summit for Stem Cell, and Aspen Neuroscience will be launching the first brain organoids to study neurodegenerative disease in microgravity to the International Space Station (ISS) on SpaceX 18.
The launch is scheduled July 21 at the Kennedy Space Center in Cape Canaveral, FL. This is a preliminary flight in preparation for a first-in-kind study of neurodegeneration in microgravity scheduled to launch to the ISS later this fall.
The full research project will send patient-derived human 3D models of Parkinson’s disease (PD) and primary progressive MS (PPMS) to the ISS for a 30-day stay later this year.

Popular story: Revamp your patient handoff
A practice wanted to improve patient transfer of care within the office. A streamlined patient handoff led to better HIPAA compliance, increased professionalism, higher revenue, and more. Find out how this practice did it.
Read more!
CDC calls for STD screenings to be standard part of medical care
A surge of sexually transmitted diseases (STDs) endangers the health of many people in the U.S., according to the Centers for Disease Control and Prevention (CDC).
From 2013 to 2017, syphilis cases nearly doubled, gonorrhea cases increased by 67 percent, and chlamydia cases remained at record highs. To reverse those trends, the CDC is calling on doctors to make STD screening and timely treatment a standard part of medical care.
In response, the American Optometric Association (AOA) Health Policy Institute developed a new brief: The Role of Doctors of Optometry in the Identification, Treatment and Prevention of Sexually Transmitted Diseases (STDs).
The brief identifies ODs as a cohort of primary-care physician providers encouraged to make STD screening and timely treatment a standard part of medical optometry practice.
ODs can detect ocular signs of each of these STDs through standard in-office clinical procedures, which, in turn, provides an opportunity for earlier STD diagnosis and improved access to necessary treatments, according to the HPI brief.

Popular story: What ODs need to know about YAG laser vitreolysis for floaters
For some patients, a floater can cause a significant disturbance. In this month’s Defocus Media podcast, one OD shares how to discuss vitreolysis as a form of treatment for curious patients
Find out how!
AAOP announces 2019 recipients of Joe and Janet Barr Early Career Cornea and Contact Lens Research Award
The American Academy of Optometry (AAOP) Foundation announced the recipients of the Joe and Janet Barr Early Career Cornea and Contact Lens Research Award.
In conjunction with the joint meeting of AAOP and the World Council of Optometry (WCO), two awards will be given this year to include students abroad.
This year’s awardees are Pabita Dhungel and Rabia Mobeen.
Pabita Dhungel is an MS/PhD candidate at Pacific University College of Optometry. Her project is Impact of Scleral Lens Wear on Intraocular Pressure and Posterior Ocular Perfusion.
Rabia Mobeen is a PhD scholar at the University of New South Wales School of Optometry and Vision Science.
Her project is Corneal Immune Response in Children, Adolescents, and Adult Contact Lens Wearers.
The award is intended to provide a MS, PhD, vision science, or physiological optics student attending a school or college of optometry seed funding for a research project in cornea/ocular surface or contact lenses.
Pabita and Rabia will each receive a $2,000 award and a $750 travel fellowship to attend Academy 2019 Orlando and 3rd World Congress of Optometry, Oct. 23-27.
This week in optometry news: July 1, 2019
VSP enters definitive agreement to acquire Visionworks

VSP Global announced that it has entered into a definitive agreement to acquire Visionworks, subject to completion of regulatory approval.
With a footprint of more than 700 stores in nearly 40 states, the Visionworks acquisition will be the single largest VSP network investment in the company’s 65-year history, according to the company.
“This transaction is highly complementary to our business and marks a significant leap forward in continuing to fulfill our vision to provide access to affordable, high-quality eye care and eyewear to more people,” says Michael Guyette, president and CEO of VSP Global. “With expanded nationwide access, we’ll provide our clients and members with an option for a more substantial, consistent and sustainable retail experience, further enhanced by the professional care of VSP network doctors.”
The transaction will close as soon as regulatory approvals are obtained.

This week’s most popular story: What ODs need to know about YAG laser vitreolysis for floaters
For some patients, a floater can cause a significant disturbance. In this month’s Defocus Media podcast, one OD shares how to discuss vitreolysis as a form of treatment for curious patients
Find out how!
Eyeconic launches first two brick-and-mortar stores in Chicago

VSP Global has entered brick-and-mortar retail with the opening of the company’s first two Eyeconic stores in the country. The stores will serve as an extension of VSP’s ecommerce site.
Each Eyeconic store will feature a selection of 500 men’s, women’s and kids’ frames from over 30 brands that span fashion, lifestyle, and performance categories such as Calvin Klein, Nike, Salvatore Ferragamo, DKNY, and Cole Haan.
Bundled frame and lens packages offer transparent pricing, and sales associates will not work on commission, according to the company. The stores will also offer all major brands of contact lenses.
Patients can schedule eye exams online and browse eyewear before coming in to see a VSP network doctor. Aisle integration with virtual try-on at eyeconic.com includes frame options from over 60 brands and 2,300 styles.
Eyeconic stores are considered in network for most VSP, Cigna, and MetLife members. Eyeconic also accepts private pay and most other vision insurance plans as an out-of-network option.
The first Chicago store, located at 1647 North Damen Ave., opened to the public on June 15. The second store, located at 916 W. Randolph St., will open to the public on July 13 and host a grand opening celebration on July 27.

Top-read story: Blog: Mission trips make an impact
An OD shares her experience traveling to the Philippines on a mission trip to provide eyecare screenings and free glasses to patients in need.
Read more!
Alcon launches Dailies Colors contact lenses
Alcon announced the debut of Dailies Colors contact lenses that combine eye color enhancement with daily disposable contact lens.

The new contact lenses deliver color enhancement by combining an eye-defining outer ring with Alcon’s three-in-one color technology:
• Inner ring, which brightens and adds depth
• Primary color, which enhances eye color
• Outer ring, which defines and emphasizes iris
In celebration of the launch, Alcon partnered with celebrity makeup artist Patrick Ta to create a collection of eye styles inspired by the new Dailies Colors and existing Air Optix Colors shades. The looks will appear on the brands’ social media platforms throughout the year.
Consumers interested in enhancing their eye color can visit the virtual Colors Studio to upload a photo and “try on” a total of 16 colors across the Dailies Colors and Air Optix Colors portfolios.

Popular story: I just graduated from optometry school … now what?
Graduating from optometry school is only the beginning of a career, and big decisions loom large. Check out this advice for new grads.
Read on!
FDA approves first drug for neuromyelitis optica

The U.S. Food & Drug Administration approved Soliris (eculizumab, Alexion Pharmaceuticals) injection for intravenous use for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adult patients who are anti-aquaporin-4 (AQP4) antibody positive.
Individuals with NMOSD typically have attacks of optic neuritis, which causes eye pain and vision loss.
The effectiveness of Soliris for the treatment of NMOSD was demonstrated in a clinical study of 143 patients with NMOSD who had antibodies against anti-AQP4 positive (AQP4) who were randomized to receive Soliris treatment or a placebo.
Compared to treatment with placebo, the study showed that treatment with Soliris reduced the number of NMOSD relapses by 94 percent over the 48-week course of the trial, according to the company. Soliris also reduced the need for hospitalizations and for treatment of acute attacks with corticosteroids and plasma exchange.
Patients should be monitored for early signs of meningococcal infections and evaluated immediately if infection is suspected. Use should be discontinued in patients who are being treated for serious meningococcal infections. In the NMOSD clinical trial, no cases of meningococcal infection were observed.
Top story: Branded vs. generics: You make the call
Educating patients about potential differences between branded and generic medications, becoming informed about their out-of-pocket costs, and following up to make sure that patients are using the intended medication is time consuming but important.
Learn why!
Outdoor exercise reduces progression of common vision issue in children
New research suggests that adding 30 minutes of daily outdoor activity reduces the progression of myopia in children if the activity is continued.
The study, conducted by researchers in Beijing, is published in the May 2019 issue of Translational Vision Science & Technology (TVST).
Scientists studied 382 children ages 6 and 7 at two Beijing-area schools for one year in a prospective interventional study. Students in the study group jogged for 30 minutes outdoors daily. The control group did not add this extra outdoor activity to their schedules.
Examinations at the end of one year showed that students in the study group without myopia at the baseline had lower incidence of myopia compared with students in the control group. Students with myopia at baseline also showed slower progression of myopia compared with students in the control group.
Annual follow-up exams following the conclusion of the one-year study showed that in Year Four, incidence of myopia was similar among the study and control groups.

Top story: All about red caps: Mydriatics and cycloplegics
Do you ever wonder what the difference is among all those bottles with the red caps you use in your office for dilating patients? Each has a different name and different concentration-but as far as you can tell, they all serve the end purpose of dilating patients.
Learn more!
IOT’s Steady Methodology technology receives US patent
IOT received a U.S. patent for Steady Methodology, used to create progressive lenses with reduced peripheral mean sphere power.
Steady Methodology, available only on IOT’s Camber Steady lenses, addresses unwanted changes in sphere power in the lateral portions of the lens in addition to cylinder error.
Power maps demonstrate how Steady Methodology virtually eliminates positive mean power, which is associated with defocus, in the lateral portion of the lens, according to the company.
In wearer trials conducted by IOT, the introduction of Steady Methodology resulted in an observed 14-percent increase in the width of the distance visual field.
Camber Steady lenses also feature a lower level and smooth distribution of unwanted cylinder error. This, along with the reduced mean sphere, reduces swim effect. The result is a lens with better image stability for the wearer, according to the company.

Popular story: Assessing visual crowding and its impact on glaucoma patients
A small-scale pilot study looked at the effect of visual crowding on the functionality of glaucoma patients. One OD explains why what researchers found has a significant impact on this population’s everyday life.
Read why!
Thema Optical debuts new biometric technology
Thema Optical announced the U.S. debut of Virtual Eyewear Assistant (VEA), a new biometric technology that lets consumers custom-design eyewear manufactured to their unique facial measurements.
VEA’s 3D camera scans and measures over 500,000 biometric points on a customer’s face in less than 20 seconds, according to the company, creating a 3D facial rendering. The customer can then use the rendering to virtually try on more than 1 million style and color combinations in Thema’s iGreen Hi-Tech collection of eyewear.
Thema manufacturers a customer’s frames to the exact specifications of the facial measurements in the customer’s preferred style, color, and overall design. Turnaround time of completion is between three to five business days, according to the company.
VEA is available at Edward Beiner Optical at Mary Brickell Village in Miami with plans to roll it out to additional Edward Beiner locations in Florida later this year.
VEA will debut in Chicago and Boston later this summer and in Washington, D.C., in the fall. The technology is also available at more than 200 stores throughout Italy, where it debuted earlier this year.
This week in optometry news: June 26, 2019
AOA 2019 News roundup
Bausch + Lomb launches Ultra Multifocal for Astigmatism contact lenses in US
Bausch + Lomb announced the U.S. launch of Ultra Multifocal for Astigmatism contact lenses. The launch makes for the first and only multifocal toric lens available as a standard offering in eyecare professionals’ fit set, according to the company.
The new monthly silicone hydrogel lens combines the company’s 3-Zone Progressive multifocal design with the OpticAlign toric design. This enables eyecare professionals to provide same-day fitting during patients’ initial lens exam.
This week’s most popular story: 4 uses for OCT in OD practices
The use of anterior segment OCT in clinical practices has enhanced ODs’ abilities to treat a wide range of patient demographics.
Find out how!
EyecareLive unveils updated telemedicine platform
EyecareLive announced the release of their latest update: EyecareLive 2.0.
EyecareLive was built by and for doctors, with a mission to strengthen the doctor-patient relationship by providing a comprehensive telemedicine platform that enhances patient care, according to the company.
The launch features EyecareLive's proprietary messaging system, ECLink, which allows for quicker and more secure Health Insurance Portability and Accountability Act (HIPAA)-compliant doctor-patient communication, as well as functionality for group messaging and doctor-staff messaging.
“Our improved platform integrates with key optometry EHR systems and enables doctors to satisfy billing requirements for virtual patient encounters,” says Raj Ramchandani, CEO of EyecareLive.
The platform allows practitioners to collect fees and copays with benefit verification on an integrated payment system, according to Ramchandrani.
EyecareLive is also unveiling a new patient app, which includes several tests for monitoring the health of patient's eyes.
“By facilitating remote treatment and diagnosis of conditions like dry eye, patients can track their progress and seamlessly transmit that information to their doctor. Even contact lens patients can benefit and see more successful outcomes,” says Moshe Mendelson, OD, MBA, cofounder of EyecareLive.
Top-read story: Pros and cons of private equity firms in practice transitions
Is the traditional sale of a private practice to a new owner in danger of extinction? Private equity firms offer a new option for ODs looking to transition in or out of practice ownership.
Find out the advantages and downsides!
ATA seeks public comment on newest edition of guidelines
The American Telemedicine Association (ATA) announced it is seeking public comment on the new edition of its Ocular Telehealth-Diabetic Retinopathy Practice Guidelines. The third edition reflects new evidence, emerging technologies, and the expanded scope of ocular telehealth.

The ATA guidelines describe evidence-based best practices for ocular telehealth and are based on the accumulated knowledge and experience of eye care and telemedicine professionals, ATA special interest workgroups, and other stakeholders, according to the ATA.
The ATA believes that these guidelines will help to increase access to personalized, quality, efficient, and cost-effective remote evaluation, diagnosis and management of diabetic retinopathy (DR), and facilitate integration of diabetes eye care with primary and specialty medical care.
The guidelines include recommendations for designing, implementing, and sustaining an ocular telehealth care program, and highlights current clinical, technical, and administrative issues that form the basis for evaluating DR with telehealth services and technologies.
The ATA is seeking public comments by July 19.
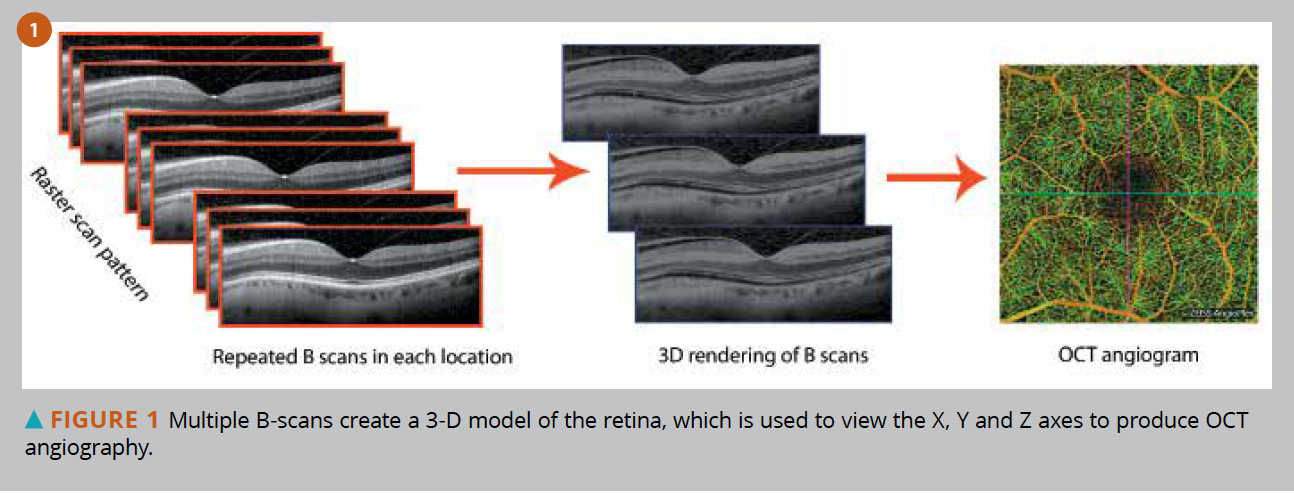
Popular story: What OCTA show us
Optical coherence tomography angiography (OCTA) has ushered in a different way for eyecare professionals to view retinal and choroidal vasculature. Find out why understanding this technology is the key to capturing images.
Find out!
Colorlite’s Colorblind Diagnositc and Correction System available in US
Widely used in Europe for more than 20 years, Colorlite’s Colorblind Diagnostic and Correction System is now available in the U.S.
Over 95 percent of Colorlite patients pass the Ishihara color blindness test, the company reports. The system is registered with the Food and Drug Administration (FDA).
The company advises colorblind people to visit their nearest Colorlite affiliate to receive a color vision diagnostic exam. Based on the results of that exam, the doctor will write a prescription for color vision correction glasses. The lenses are available with or without a vision prescription and can be cut to fit most frames.
Top story: Case report: Don’t be misled by diabetic retinopathy
Patients with diabetes are at risk for developing diabetic retinopathy and potentially blindness. Case example illustrates how proper treatment makes a difference.
Read more!
This week in optometry news: June 26, 2019
CooperVision report reveals factors behind widespread shift to 1-day contact lenses
A new industry report from CooperVision profiles the beliefs and behaviors driving the switch to daily disposable contact lenses from monthly and two-week modalities.

Based on surveys conducted among 450 eyecare professionals (ECPs) and 2,000 contact lens wearers in the U.S., Germany, Spain, Italy, and France, the findings showed professional actions corroborated by scientific evidence. However, it also revealed challenges caused by perceived barriers to adoption.
The latest publication in the company’s consumer insights series, the report is available for online review and download from coopervision.com/frp-to-1day-sihy.
Five primary insights are highlighted in the new publication, each providing a viewpoint on daily disposable refitting trendlines:
1. The pace of 1-day refitting is increasing. Compared to six months ago, 79 percent of U.S. ECPs and 46 percent of European ECPs say they are moving more FRP wearers to daily disposable, with a similar proportion feeling this trend will continue over the next year.
2. ECP recommendations matter most. In 84 percent of all switching scenarios, the ECP is driving the change. Moreover, 69 percent of patients report that they make their final lens choice based on the ECP recommendation.
3. Eye health and comfort are behind the shift. In 70 percent of switching cases, ECPs suggested the change because of patient concerns with previous contact lenses. Professionals cited general and long-term eye health, comfort at the end of the day, and better fit for patient lifestyle as benefits of moving to a daily disposable modality.
4. Beliefs don’t always equate to action. Despite nearly nine in 10 ECPs saying that they believe “those in 1-day lenses should be in a silicone hydrogel material,” it is surprising that nearly half of patients moved from FRP silicone hydrogel lenses are fit with daily disposable hydrogel materials. This disconnect exists even though more than two-thirds of contact lens wearers say they expect their ECP to recommend lenses-regardless of the cost-that provide the oxygen their eyes need.
5. Misperceptions fuel ECP hesitancy. When asked about their use of a hydrogel material when refitting wearers into a daily disposable cotact lens, 55 percent of ECPs cited price concerns, while 28 percent of ECPs believe some patients do not wear their lenses enough to require the oxygen transmissibility benefit of silicone hydrogel materials.

Top story: How to educate patients on risks of eyelash enhancements
Fake eyelashes are a growing beauty trend among makeup consumers across the globe. But ocular surface complications can arise when proper management and treatment are not practiced.
Learn more!
RightEye partners with USA Baseball to improve athletes’ sports vision

RightEye LLC announced that it is working with USA Baseball for the third consecutive year to provide baseline sports vision assessment to all athletes entering the Prospect Development Pipeline (PDP), a collaboration between USA Baseball and Major League Baseball (MLB).
For the first time, RightEye will also recommend local ODs who can help athletes correct functional vision problems and improve performance on the field.
At 34 PDP events taking place across the country, prospective collegiate and professional baseball players will be screened with RightEye Sports Vision and Functional Vision EyeQ. RightEyeEyeQ provides insight into an athlete’s visual and eye processing strengths and areas that can be improved upon in order to reach maximum performance levels.
In addition to sports vision, any concerns found with an athlete’s functional vision, including eye alignment and teaming, object tracking, depth perception, and dynamic visual acuity, will be identified. RightEye will recommend athletes to local ODs in their region who can provide customized vision training programs designed to improve their functional vision, in addition to sports performance.

Popular story: All about red caps: Mydriatics and cycloplegics
Do you ever wonder what the difference is among all those bottles with the red caps you use in your office for dilating patients? Each has a different name and different concentration-but as far as you can tell, they all serve the end purpose of dilating patients.
Read more!
CORE develops ‘5-minute overviews’ of IMI’s white papers, clinical guidance

The International Myopia Institute (IMI) has announced plans to continue disseminating results of its recent white papers, including developing short summaries, according to a June update to its members.
Centre for Ocular Research & Education (CORE) has published “5-minute overviews” of each paper ahead of the official release, authored by the CORE clinical science team.
In conjunction with Kate Gifford, OD,-cofounder of Myopia Profile, a resource on clinical myopia management for eyecare professionals-CORE has also created a downloadable set of practical tips on how to implement the clinical management guidelines in practice.
The summaries, reference guide, and additional myopia management insights can be accessed for free online.
The summaries and clinical insights fact sheet are part of a special edition of Contact Lens Update, which provides a global platform for unbiased clinical insights based in current research.
Since 2011, each issue has provided up-to-date ocular health information for more than 60,000 leading eyecare professionals. The publication receives support from the educational arms of Alcon and Johnson & Johnson Vision.
Popular story: Telephone triage: Recognize potential visual disturbance emergencies
All staffers who interact with patients must be able to recognize possible emergencies of visual disturbance. Know when a patient must come in immediately, can wait until tomorrow, or can be scheduled for the next available appointment.
Learn more!
First pre-pilot clinical from Ocutrx Vision Technologies improves vision in AMD patients
Ocutrx Vision Technologies, LLC, announced the results of the first pre-pilot clinical trial for the company’s Oculenz AR glasses.
In the single arm crossover study of subjects with advanced macular degeneration (AMD), the Oculenz AR glasses significantly improved the ability of subjects to read small letters and print at near and far viewing distances when compared to their own standard near correction (or no correction) in subjects with scotoma impairment from AMD, according to the company.
The trial subjects initially used a self-calibration module where the digital AR headset identified the subject’s scotoma (scotoma marker), which correlated the area of non-vision with the AR headset.
The self-calibration results were compared to a fundus autofluorescence (FAF) photo of each eye taken prior to the testing. Reading performance was evaluated using the logMAR chart, per eye, both before and after wearing the Oculenz AR headset.
The CNEC was used to test reading skills before and after wearing the Oculenz device set at no magnification. The study consisted of one visit, which included FAF photos, training with the Oculenz headset, and visual function tests.
Mean age of the study’s subjects was 68 (54-76). The best-corrected acuity was from less than 20/200 with near (or no correction) improving to 20/63 with the Oculenz ARwear without magnification. Mean critical print size (CPS) improved from 0.96 logMAR (L/R) with near or no correction to 0.61 (L) and 0.58 (R) logMAR with the Oculenz ARwear at no magnification. Improvement ranged up to five lines on the logMAR chart without magnification.
All subjects had difficulty reading, and most subjects had not been able to read for years prior to wearing Oculenz. Participants were able to read at a standard reading pace at 30-point type (J-16), with some reading down to 12-point type (J-10) in just one session wearing the Oculenz.
These results suggest that with “neuro-adaptation,” subjects learned to comprehend the entire modified view and ignore the blind spot in reading.
The Oculenz headset provides a “perceived de-emphasis” of the scotoma and enhanced reading of the letters and letters in a word, as well as enhanced recognition of faces and features of familiar individuals, according to the company.
Popular story: Troubleshooting optical complaints
There is no doubt that optical errors adversely affect patient satisfaction and practice success. The best way for technicians and opticians to differentiate whether the patient actually needs to see the doctor for a re-check is to go through an optical checklist.
Read on!
EyePromise introduces Screen Shield Pro as screen time protection for adults
EyePromise introducd Screen Shield Pro to its premium eye vitamin line. Screen Shield Pro is designed to protect and relieve digital eye strain symptoms of adults (ages 18 and up) who spend eight or more hours staring at digital devices.
This follows the recent introduction of Screen Shield Teen, an ocular nutrition supplement formulated for visual comfort and wellness for children ages 4 to 17. Screen Shield Pro makes EyePromise the first to develop an eye vitamin for ages 4 and up that naturally defends eyes from screen time, according to the company.
The supplement is made with zeaxanthin, lutein, omega-3s, and other nutrients that positively impact symptoms associated with increased screen time, according to the company.
This week in optometry news: June 17, 2019
California budget restores crucial Medi-Cal eyeglass benefit
The California Optometric Association (COA) released the following statement from President Ronald G. Seger OD, FAAO, in response to the 2019 -2020 state budget passed by the legislature on Thursday, which is expected to be signed by Gov. Gavin Newsom:

“California’s doctors of optometry applaud Gov. Newsom and California’s legislators for recognizing the opportunity to improve sight and save lives by restoring eyeglasses and vision aid coverage for adults participating in Medi-Cal,” Dr. Seger says. “We are proud to have worked alongside patient advocates to raise this issue to the forefront of budget discussions with the goal of increasing early detection, connecting patients with care needed to manage conditions like diabetes and heart disease, and preventing severe systemic complications ranging from blindness to amputations,” he says.
Gov. Newsom’s original budget plan-unveiled in January-proposed a broad overhaul of health care: lowering prescription drug costs, expanding Obamacare so middle-class families can receive subsidies to buy insurance, and offering Medi-Cal coverage to undocumented immigrants up to age 26. The revised budget was submitted based on the latest economic forecasts.
The revised budget includes $162.3 billion for all health and humans services-an increase of $1.1 billion in general funding compared to the governor’s original budget.
“ODs have a clear, 20/20 vision that integrates eye care into a healthcare system focused on prevention to bridge health care gaps for disadvantaged communities,” Dr. Seger says. “This state budget investment is an important step forward to improve sight and create healthy lives,” he says.

This week’s most popular story: How to educate patients on the risks of eyelash enhancements
Fake eyelashes are a growing beauty trend among makeup consumers across the globe. But ocular surface complications can arise when proper management and treatment are not practiced.
Learn more!
Prevention Bio's Teplizumab delays onset of Type 1 diabetes, trial results show
Provention Bio, Inc. announced that results from the National Institutes of Health (NIH)-sponsored study were published online in The New England Journal of Medicine and presented at the Scientific Sessions of the 79th Annual American Diabetes Association (ADA) meeting.
The study was conducted by Type 1 Diabetes TrialNet, which evaluated Provention's PRV-031 (teplizumab) for the prevention or delay of clinical type 1 diabetes in relatives of type 1 diabetics at high-risk of developing the disease.
The "at -risk" study enrolled 76 participants ages 8 to 49 who were "At-Risk" because they had two or more type 1 diabetes autoantibodies and abnormal glucose metabolism (dysglycemia); 72 percent of participants were under the age of 18. Subjects were randomized to receive either teplizumab or placebo.
Results from the study showed that a single 14-day course of teplizumab significantly delayed the onset and diagnosis of clinical type 1 diabetes, as compared to a placebo, by a median of two years in children and adults considered to be at high risk.
The median time to clinical diagnosis of type 1 diabetes for placebo participants was just over 24 months.
In comparison, the median time for teplizumab-treated participants to clinical diagnosis of T1D was just over 48 months (p=0.006). During the trial, 72 percent in the placebo group developed clinical diabetes compared to only 43 percent of the teplizumab group. Teplizumab was well tolerated and the safety data were consistent with prior studies in newly diagnosed patients.

Top-read story: Know the connection between vitamin D and dry eye disease
Epidemiological studies have linked vitamin D deficiency with ocular diseases, yet the underlying mechanism is not yet fully understood. Conflicting data finds a possible connection to DED patients.
Read more!
Study: Concussions are leading cause of injury for children in recreational sports
Elementary school-aged children who participate in recreational sports are at greater risk of concussion than most other sports-related injuries. A new study published in PLOS ONE focused on children 5 to 11 years old who play recreational football, soccer, and baseball/softball.

Karen Liller, PhD, professor of community and family health at the University of South Florida College of Public Health, followed more than 1,500 athletes each year for two years in Hillsborough County, FL.
She and her colleagues collected baseline neurocognitive data using ImPACT Pediatric, the only Food and Drug Administration (FDA)-approved concussion assessment tool for ages 5 to 11.
The digital program asks athletes questions pertaining to word memory, sequencing/attention, visual memory, and reaction time. It was administered prior to practice and games to help prevent fatigue from impacting test performance.
Certified Athletic Trainers (ATCs) were hired to collect injury data using High School Reporting Information Online (RIO), an internet-based injury surveillance system.
During the two-year study, 26 athletes were injured-12 were diagnosed with a concussion. Of those concussions, 10 occurred during soccer, and the remainder took place during softball games.
In addition to noting specific injuries, the RIO records how frequent each athlete participates in a sport, where the individual is located, what he was doing when he got hurt, and exactly how it happened.
Researchers found the leading mechanisms of injury were caused by colliding with another athlete, contact with a playing apparatus, and contact with playing surfaces. While none of the injuries required surgery, they did result in lost playing time for an athlete.
The ATCs conducted follow-up assessments for ImPACT Pediatric on the athletes diagnosed with concussions. They found similar findings to baseline levels when the athletes returned to play.

Popular story: How to control myopia progression in your practice
As the number of myopic patients increases across the globe, ODs need to know how to diagnose and slow the progression of myopia. Find out how one OD handles cases in her practice.
Find out!
EyePACS surpasses 5 million diagnostic retinal images in comprehensive database
EyePACS LLC announced that it surpassed 5 million retinal images in its global comprehensive retinal diagnostic and study database this past February.
The EyePACS telemedicine platform is the largest assessment network of its kind for diabetic retinopathy.
Beginning in 2003, the EyePACS comprehensive retina diagnostic and study platform has been used by certified and credentialed eye clinicians to provide retinal assessment for nearly 1 million diabetic patients.
The platform has been deployed in more than 600 community health centers across the U.S. and additional sites internationally.

Top story: Using warm compresses to treat meibomian gland disease
Warm compresses are commonly recommended as supplementary therapy for MGD. Providing specific instructions for a self-administered efficacious warm compress can greatly improve the standard of care offered to patients.
Learn more!
NORA, VEDA offer new educational resource on vestibular-vision connection
Vision plays a significant role in people’s ability to balance, orient ourselves in space, and process movement of things in the environment.

The vestibular (inner ear balance) system and the visual system coordinate with each other through brain pathways in order to control the eyes’ ability to maintain a visual gaze on a single location.
This connection, known as the vestibulo-ocular reflex, has a critical role in producing eye movements and stabilizing the image during head motion and helping to maintain balance.
“If the vestibular system is damaged by disease, aging, head injury, or sometimes for no apparent reason, persons with a vestibular disorder often experience extreme difficulty with balance and movement, as well as with their perception of space,” says Cynthia Ryan, executive director of the Vestibular Disorders Association (VeDA).
“A regular eye exam may not reveal the extent that the visual process is affected,” says Shirley Ha, OD, FCOVD, Advisory Board member of the Neuro Optometric Rehabilitation Association (NORA). “Since both the vestibular and visual systems are affected in spatial orientation, visual deficits related to a vestibular disorder should be evaluated by a neuro-optometric rehabilitation optometrist.
Other healthcare professionals such as neurologists, otolaryngologists, and neurotologists may also become involved in the diagnosis, treatment, and management of dizziness and balance disorders.
To assist individuals who may be experiencing visual dysfunctions contributing to dizziness and balance problems, NORA and VeDA have developed The Vestibular-Vision Connection.
This patient-education resource can be viewed and downloaded on NORA’s Patient Caregivers Resource page and VeDa’s Vision & Hearing page.
Both organizations offer additional resources to help patients and caregivers find the help they need, including search features to find providers who can help with diagnosis and treatment.

Top story: Troubleshooting optical complaints
There is no doubt that optical errors can adversely affect patient satisfaction and practice success. The best way for technicians and opticians to differentiate whether a patient actually needs to see an OD for a re-check is to go through an optical checklist.
Learn more!
Prevention Bio's Teplizumab delays onset of Type 1 diabetes, trial results show
Provention Bio, Inc. announced that results from the National Institutes of Health (NIH)-sponsored study were published online in The New England Journal of Medicine and presented at the Scientific Sessions of the 79th Annual American Diabetes Association (ADA) meeting.
The study was conducted by Type 1 Diabetes TrialNet, which evaluated Provention's PRV-031 (teplizumab) for the prevention or delay of clinical type 1 diabetes in relatives of type 1 diabetics at high-risk of developing the disease.
The "at -risk" study enrolled 76 participants ages 8 to 49 who were "At-Risk" because they had two or more type 1 diabetes autoantibodies and abnormal glucose metabolism (dysglycemia); 72 percent of participants were under the age of 18. Subjects were randomized to receive either teplizumab or placebo.
Results from the study showed that a single 14-day course of teplizumab significantly delayed the onset and diagnosis of clinical type 1 diabetes, as compared to a placebo, by a median of two years in children and adults considered to be at high risk.
The median time to clinical diagnosis of type 1 diabetes for placebo participants was just over 24 months.
In comparison, the median time for teplizumab-treated participants to clinical diagnosis of T1D was just over 48 months (p=0.006). During the trial, 72 percent in the placebo group developed clinical diabetes compared to only 43 percent of the teplizumab group. Teplizumab was well tolerated and the safety data were consistent with prior studies in newly diagnosed patients.

Popular story: How artificial intelligence is changing the future of optometry
In the age of increasing technology advancements, AI is altering ODs’ traditional models of the doctor-patient relationship.
Find out!
OCuSOFT introduces new lid scrub allergy eyelid treatment
OCuSOFT Inc. announced the availability of OCuSOFT Lid Scrub Allergy Eyelid Cleanser for allergy conditions in pre-moistened pads.
OCuSOFT Lid Scrub Allergy removes oil, debris, pollen, and other contaminants from the eyelids while utilizing green tea extract, tea tree oil and PSG-2 (phytosphingosine) to effectively reduce redness, inflammation, and itching of allergy eyelids.
Clinically proven PSG-2 is known to prevent loss of moisture from the skin, regulate epidermal cell growth, differentiation and apoptosis, and possess bactericidal and anti-inflammatory properties.
When combined with green tea extract having anti-allergenic properties, tea tree oil, and mild OCuSOFT Lid Scrub eyelid cleansers, OCuSOFT Lid Scrub Allergy cleans, soothes, calms and moisturizes inflamed, irritated eyelids.
Weekly news roundup: June 10, 2019
Eyenovia enrolls first patient in Phase III study for progressive myopia
Eyenovia, Inc. announced that it has initiated its MicroPine Phase III program and enrolled the first patient in its CHAPERONE study.
The CHAPERONE study is a U.S.-based, multi-center, randomized, double-masked trial that will enroll more than 400 patients between the ages of 3 and 12.The study will investigate the safety and efficacy of MicroPine for the reduction of progressive myopia using Eyenovia’s atropine topical micro-formulation delivered by the Optejet dispenser.
Subjects will be randomized to receive treatment with two MicroPine concentrations or a placebo. The primary endpoint of the study is the change in refractive error from baseline through 36 months.
Pamela Gallin, MD, FACS, a clinical professor of ophthalmology in pediatrics and director emeritus at NY Presbyterian-Columbia University Medical Center, says the program will evaluate the benefit of slowing the progression of myopia with a microdose of low-concentration atropine.
“This may reduce the problems associated with high myopia, including a range of retinal complications,” she says.

This week’s most popular story: Blog: How I learned to stop worrying and love refractions
One OD explains why training and delegating technicians to perform refractions works at his clinic, and why doing so isn’t the end of the world for ODs.
Learn how!
Allegro Ophthalmics announces positive study results for treatment of dry AMD
Allegro Ophthalmics, LLC announced positive topline results of its Phase II study of risuteganib (Luminate) for the treatment of intermediate nonexudative age-related macular degeneration (dry AMD).
The trial was a prospective, randomized, double-masked, placebo-controlled, multi-center U.S. study that evaluated the safety and efficacy of risuteganib in patients with intermediate dry AMD.
The primary endpoint of the study was the proportion of subjects with a ≥8 letters of vision gain with two risuteganib injections versus one sham treatment.
The clinical trial met its primary endpoint with 48 percent of patients in the risuteganib arm gaining ≥8 letters of vision at Week 28 compared to baseline.
At baseline, 40 patients were randomized to receive either intravitreal 1.0 mg risuteganib or sham injection. At Week 16, patients in the risuteganib arm received a second dose of 1.0mg risuteganib, and patients in the sham arm crossed over and received a single dose of 1.0mg risuteganib.
The primary endpoint was the percentage of the population with ≥8 letters Early Treatment Diabetic Retinopathy Study (ETDRS) best corrected visual acuity (BCVA) gain from baseline to week 28 in the 1.0 mg risuteganib arm versus from baseline to week 12 in the sham arm.
The primary endpoint was prespecified as ≥ 8 letters to account for the variability in visual acuity measurements among patients with intermediate dry AMD. Forty-eight percent of patients in the risuteganib arm at Week 28 and 7 percent of patients in the sham group at Qeek 12 gained ≥8 letters from baseline (p=0.013).
Risuteganib was found to be safe, with no reported drug-related serious adverse events (SAEs), according to the company.
Secondary outcomes-including microperimetry, color vision, and low luminance visual acuity-are currently being evaluated. Results will be released in the upcoming weeks.
Top-read story: Blog: A different approach to treating dry eyes in Japan
Margie Recalde, OD, FAAO, takes a look at the contrasting approaches eyecare professionals in the U.S. and Japan have to dry eye treatment.
Find out how they compare.
New rosacea survey shows positive impact of clear skin
A recent National Rosacea Society (NRS) survey found that highly successful medical treatment for rosacea often has a major positive impact on patients’ lives.
In the survey of 1,044 rosacea patients, around 76 percent of respondents saw at least some improvement in their skin after receiving treatment.
Among those patients, 40 percent said that treatment had improved their psychological well-being, 35 percent said their social well-being had improved, and 31 percent saw improvement in their occupational well-being.
When the signs and symptoms of rosacea are virtually eliminated, however, the improvement in patients’ lives was often dramatic.
Eighty-one percent of those who had achieved clear or almost clear skin said their psychological well-being had improved; 71 percent said it had also improved their social lives; and 62 percent reported improvement in their occupational well-being.
By contrast, among patients whose rosacea was only slightly or moderately improved, only 24 percent reported improved psychological well-being; 21 percent said they felt their social lives had improved; and 19 percent said they were better off at work.
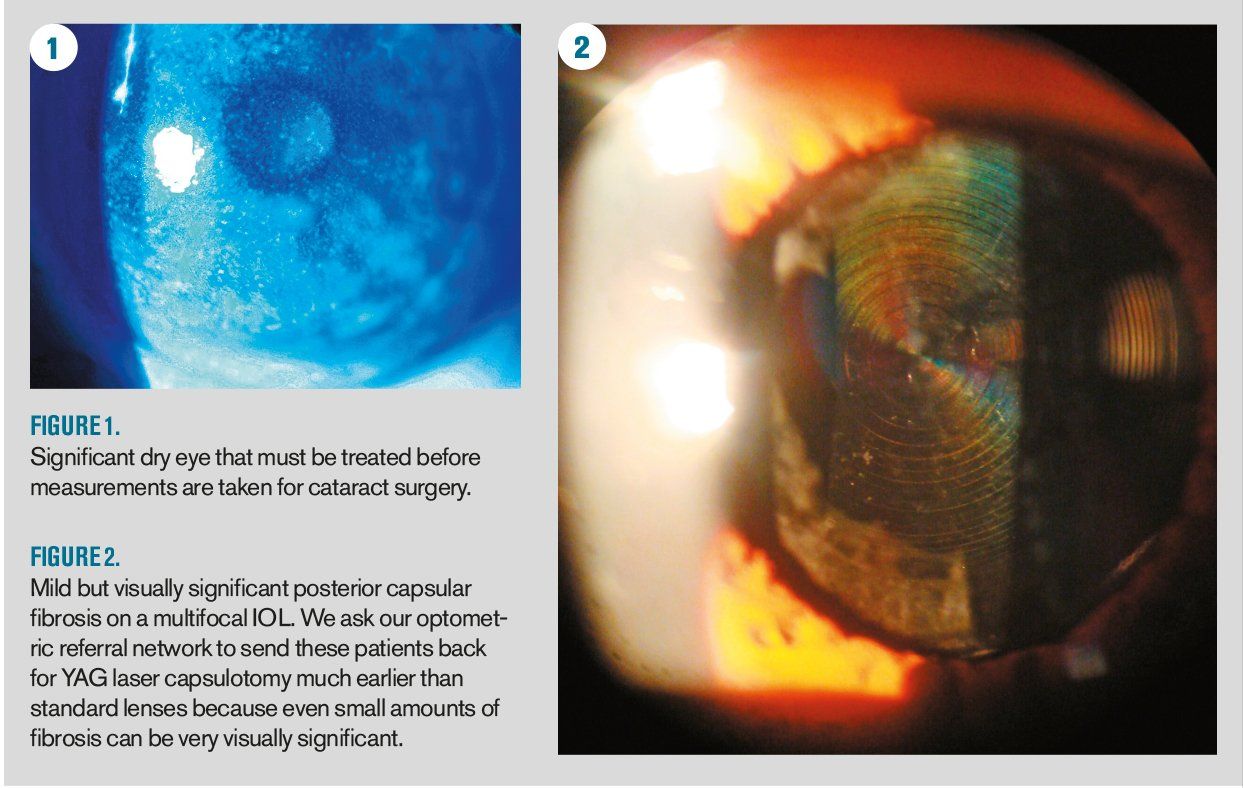
Popular story: Cataract surgery comanagement from the other
An OD working in a surgical center explains how comanagement with referring ODs occurs at his center, and why doing so can help ensure better patient outcomes.
Learn more!
M&S Technologies receives patent for clinical trial technology

M&S Technologies received a U.S. patient for a testing method that provides repeatable and accurate testing of a patient in a reduced amount of time. This allows consistent data generation from site-to-site and visit-to-visit while reducing data collection and dissemination expense.
The M&S Clinical Trial Suite (CTS) testing method includes a display, presenting the first line of optotypes where each line progressively decreases in size and in contrast of the optotype relative to the background.
A patient identifies the line of optotype in which he can correctly identify all optotypes in the first chart. A second chart is then presented, where each line gets progressively smaller. The method may include randomizing the optotypes on the second chart. The method continues until the patient is no longer able to correctly identify any of the optotypes on a line.

Popular story: I just graduated optometry school … now what?
Graduating from optometry school is only the beginning of a career, and big decisions loom large. Check out this advice for new grads.
Read on!
The Cooper Companies supports United Nations Sustainable Development Goals
The Cooper Companies, Inc. announced its alignment with the United Nations (UN) Sustainable Development Goals (SDGs), a global framework and action plan to end poverty, protect the planet, and ensure prosperity and peace by 2030.

The company has outlined key areas where it is delivering positive outcomes in line with the UN SDGs, concentrating its current efforts on three goals it believes to be most relevant for its organization and business activities:
Good health and well-being
From developing products to help improve the way people see each day to delivering solutions that better individuals’ lives, Cooper’s medical products and technical expertise help meet evolving needs in the specialty healthcare market.
Responsible consumption and production
Initiatives aimed to minimize the environmental impact of its operations include powering sites with renewable electricity, implementing production improvements to conserve water and energy, recycling materials needed to make and distribute its products, and certifying facilities to internationally recognized sustainability standards.
Partnerships for the goals
The company emphasizes that partnerships are integral to how it effectively delivers healthcare solutions, including partnering for customer success, research and development partnerships, and when working with academia and nonprofits.
This week in optometry news: June 3, 2019
Brain stimulation enhances visual learning speed and efficiency
A group of international researchers collaborated to study how different types of non-invasive brain stimulation affect visual perceptual learning and retention in both healthy individuals and those with brain damage.
Their results, published in a paper in the Journal of Neuroscience, could lead to enhanced learning efficacy for both populations and improved vision recovery for cortically blind patients.
Team members involved in this research included:
• University of Rochester researchers Krystel Huxlin and Duje Tadin, professor of brain and cognitive sciences
• James V. Aquavella, MD, professor in ophthalmology at the University’s Flaum Eye Institute
• Lorella Battelli, group leader at the Italian Institute of Technology and assistant professor at Harvard Medical School
The researchers presented study participants with a computer-based task to test if and how visual perceptual learning might be accelerated,
Participants were shown clouds of dots and asked to determine which way the dots moved across the computer screen. The task measured the participants’ motion integration threshold.
Participants were then asked to perform the task while sub-groups were given different types of brain stimulations, each involving a non-invasive electrical current applied over the visual cortex.
The researchers found that one particular type of stimulation- transcranial random noise stimulation (tRNS)-had positive effects on improving participants’ motion integration thresholds when they performed the task.
“All groups of participants improved the dot motion task with practice, but the group that also trained with tRNS improved twice as much and was able to learn the motion task better than other groups,” Tadin says.
Researchers also found that when they re-tested the participants six months later, the boosts in performance were still there: the participants in the tRNS group had retained what they had learned and were still able to do better on the motion task compared to the groups that were given other stimulation techniques or training alone.
Tadin, Huxlin, and Battelli extended the findings to patients who had suffered a stroke or other traumatic brain injury that affected their visual cortex, leaving them partially blind.
The research offers promise for overcoming key hurdles in vision therapy for patients who have experienced a stroke or traumatic brain injury. However, it is unclear how long the recovered abilities are retained once the therapy has ended.

This week’s most popular story: First impressions of Acuvue Oasys with Transitions contact lenses
The latest Defocus Media podcast and story explores what ODs need to know to position this contact lens for success in their practice and what patient expectations to manage before getting started.
Read and listen here!
DrChrono, Updox expand partnership integrating collaboration platform into HER

DrChrono Inc. and Updox announced that practices using DrChrono’s electronic health records (EHR) will receive access to Updox’s full collaboration suite. The Updox platform enables DrChrono providers to consolidate administrative tasks, patient engagement initiatives, and provider communications.
By accessing the Updox app through DrChrono’s Healthcare App Directory, practices can eliminate staff fatigue associated with manual faxing, printing and scanning, phone tag with patients and providers, and using multiple systems to execute daily tasks.
Updox’s communication platform within DrChrono’s App Directory includes document editing, one-click provider signature, tags and queues for easier triage and prioritization, and inbound/outbound fax. Engagement tools like secure text messaging, SMS Quicksend, and broadcast messaging are also included. Video chat will be added shortly.
The platform includes a Health Insurance Portability and Accountability Act (HIPAA)-compliant inbox to manage all tasks, as well as an internal chat solution for staff and providers.
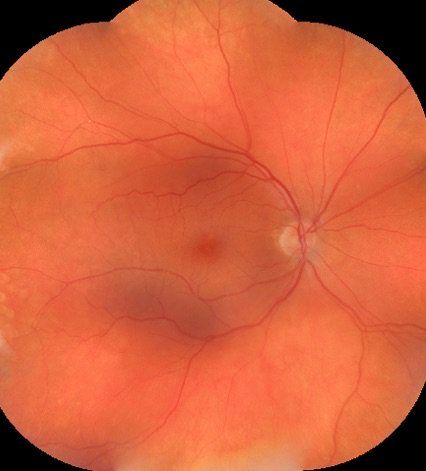
Popular story: Why Hollenhorst plaques can be key to carotid disease diagnosis
Microscopic cholesterol findings in the retinal arterioles of a 74-year-old patient lead to a diagnosis of carotid artery aneurism. Find out why the presence of Hollenhorst plaques during retinal examination played a major role.
Find out!
Johnson & Johnson Vision’s Acuvue Oasys with Transitions contact lenses hits UK market
Johnson & Johnson Vision announced that Acuvue Oasys with Transitions light intelligent technology will soon be commercially available in the United Kingdom.
The photochromic, two-week reusable contact lens is designed to adapt to changing light indoors and outdoors, help eyes recover from bright light up to 5 seconds faster, and reduce halos and starbursts around bright lights at night.
The lenses are also expected to rollout to other markets in Europe, the Middle East, and Africa soon.

Top-read story: Blog: A different approach to treating dry eyes in Japan
Margie Recalde, OD, FAAO, takes a look at the contrasting approaches eyecare professionals in the U.S. and Japan have to dry eye treatment.
Find out how they compare.
ABB Optical Group announces 6th annual ABB Cares Program
ABB Optical Group will be accepting applications for its sixth annual ABB Cares Program beginning August 1.
In the last five years, the community grants program has awarded non-profit organizations across the country more than $65,000 in grants, according to the company.
This year, the organization will award one ABB Cares Platinum Grant of $5,000, two gold grants of $2,500 each, and four silver grants of $1,000 to charities nominated by professionals in the eyecare industry. Organizations are not required to have a focus on eye health to qualify for a grant.

To be considered as a grant recipient, candidates must comply with the following:
• Organization must be a 501(c)(3) organization and provide proof of that status
• Organization's local office must be within 30 miles of the nominating practice's primary location
• Organization must be located in the U.S.
• All applications must be submitted in English
• Applications must be completed and submitted online
• Deadline to submit an application is 11:59 p.m. ET on August 31
Popular story: How private equity affects optometry
The rise in private equity in optometry has changed the playing field for ODs looking for an exit strategy as well as those interested in purchasing a practice.
Learn how.
Changes to immune genes link paternal tobacco use to childhood asthma
Children exposed to paternal tobacco smoking before birth are more likely to develop asthma- and associated changes to immune genes predict the level of risk.
These are the findings of a new study of Taiwanese families whose lifestyle and genetic makeup were analyzed to determine how fathers’ tobacco smoking during pregnancy relates to asthma risk in their children.
Published in Frontiers in Genetics, the study reinforces the risks of either parent smoking-and according to the authors, could provide DNA targets for early prediction and reversal of tobacco smoking-associated childhood asthma.
“We found that prenatal exposure to paternal tobacco smoking is associated with increased methylation of certain immune genes, which alters how the genetic code is read,” says Chih Chiang Wu, MD, of Po-Zen Hospital in Taiwan. “This smoking-associated DNA methylation is significantly retained from birth to 6 years of age, and correlates with development of childhood asthma.”
The study is the first to show that-similar to maternal smoking or air pollution-paternal smoking during pregnancy can program epigenetic modifications in important immune system genes, and that these modifications are associated with an increased risk of childhood asthma.
“Twenty-three percent of the fathers were smokers, compared to just three of the mothers,” says Ho Chang Kuo, MD, PhD, of Kaohsiung Chang Gung Memorial Hospital.

Popular story: Troubleshoot contact lens discomfort and prevent complications
Ocular surface disease can be further complicated by contact lens wear. Keep an open mind to the possible underlying conditions, even with asymptomatic patients.
Learn more!
Nidek launches YC-200 S plus/YC-200
Nidek announced the launch of its YC-200 S plus Ophthalmic YAG and SLT Laser System / YC-200 Ophthalmic YAG Laser System, according to the company.
The YC-200 S plus / YC-200 is the advanced successor to the YC-1800 laser. The YC-200 S plus / YC-200 of lasers builds on the technology of the YC-1800 by incorporating newer optical designs, engineering, and software advances to create precise targeting of pathology, ensure successful treatments, and enhance surgeon visualization of laser delivery, according to the company.
The optical system includes an expanded focal depth and natural-colored bright light-emitting diod (LED) illumination that provide views of the pathology and treatment. Two rotatable aiming beams for YAG mode and a parfocal aiming beam for selective laser trabeculoplasty (SLT) mode allows surgeons to accurately target pathology.
YAG laser system additions to the YC-200 S plus / YC-200 reach a 1.6 mJ plasma threshold in air. These improvements allow for homogeneous laser energy delivery. The YC-200 S plus offers an advanced SLT mode that includes SLT-NAVI- a display of the real-time progress of laser treatment.
This week in optometry news: May 27, 2019
BCLA unveils finalists for 2019 BCLA Awards
The British Contact Lens Association (BCLA) has announced the shortlist for the 2019 BCLA Awards.
Held as part of this year’s BCLA Clinical Conference and Exhibition, the awards will honor eyecare professionals who have a track record for excellence in eye care and a passion for pioneering new techniques.

Saleel Jivraj, OD, and Iain Johnson, LDO, are finalists for the Dry Eye Practitioner of the Year award, which aims to honor and recognize the work of an eyecare professional who has set up and runs a successful dry eye clinic.
Luke Allen, OD, and Eleanor Hill, LDO, have been shortlisted in the Young Contact Lens Practitioner of the Year category, launched to recognize the work of an eyecare professional who has been qualified for no more than five years operating within the field of contact lenses.
The shortlist for the BCLA Industry Award includes:
• Erich Bauman, OD, MBA, MA, and John Pruitt, PhD (Alcon)
• John Phillips (CooperVision)
• Stuart Cockerill, Paul Chamberlain, BSc(Hons), and Steve Newman (Menicon)
• Kurt Moody, OD, FAAO, and John Meyler (Johnson & Johnson Vision)
Additional submissions come from Zohra Fadli, PhD; Leilani Sonoda, and John Buch, MD. Rick Weisbarth, OD, FAAO, is also included on the shortlist as an individual nomination
All those shortlisted have met the criteria for nominations to have made an outstanding contribution to the contact lens profession either in relation to a product/technology or a concept.
The award is aimed to honor and recognize the entrepreneurial work being carried out by individuals or team of individuals working in contact lens science, research, and technology within the industry.
The three-day clinical conference and exhibition takes place May 30 to June 1.

This week’s most popular story: Experts offer advice on fitting, pricing daily disposables
We asked 15 contact lens experts how they would advise their colleagues in fitting daily disposable contact lenses as well as discussing price with patients.
Find out what they said!
Brien Holden Vision introduces Global Myopia Centre
Brien Holden Vision Institute’s new Global Myopia Centre combines onto one platform expertise and resources in research, innovation, and education with global advocacy efforts in myopia.
The website offers a single sign-on feature for users to create their own account. Online tools available include educational resources and infographics.
Also new to the site are feature editorials and academic blogs authored by experts in myopia management and researchers at the institute.
The new platform will host the institute’s Myopia Education Program as well as the latest in innovation and industry initiatives.

Top-read story: Blog: A different approach to treating dry eyes in Japan
Margie Recalde, OD, FAAO, takes a look at the contrasting approaches eyecare professionals in the U.S. and Japan have to dry eye treatment.
Find out how they compare.
Allergan, SightSavers, IAPB launch glaucoma initiative
Allergan, Sightsavers, and the International Agency for the Prevention of Blindness (IAPB) announced a joint initiative-“Keep Sight”-to prevent glaucoma-related vision loss by building healthcare capacity in low- and middle-income countries with the highest unmet need.
The initiative will provide training for healthcare professionals to screen at-risk populations, ensure early and accurate diagnosis, and provide appropriate treatment and long-term care in an effort to make a positive impact on people with glaucoma.
The first program will launch at a high-volume hospital in Nigeria. Training will be provided for 50 healthcare professionals with a goal of screening 5,000 people, treating 500 patients, and providing surgery for 70 patients.
The initiative is expected to expand to screen 500,000 people in high-burden countries by 2021.

Popular story: How to control myopia progression in your practice
As the number of myopic patients increases across the globe, ODs need to know how to diagnose and slow the progression of myopia. Find out how one OD handles cases in her practice.
Find out!
Prevent Blindness declares June as Cataract Awareness Month
Prevent Blindness has declared June as Cataract Awareness Month to educate the public on risk factors, symptoms, and treatment options.
Most cataracts are caused by changes related to aging. However, other factors may cause cataracts to form including eye infections, some medicines (such as steroids), and injuries. Prolonged exposure to ultraviolet light (UV) and various diseases, such as diabetes or metabolic disorders, may also contribute to cataracts forming.
Cataract symptoms may include:
• Blurred vision, double vision, ghost images, or the sense of a "film" over the eyes
• Poor night vision
• Lights seem too dim for reading or close-up work
• Eyeglass prescriptions change often
• A visible milky or yellowish spot can be seen in the pupil
A healthy lifestyle can decrease the risks of developing cataract. Quitting smoking, controlling blood sugar levels, and consistently wearing UV-protecting sunglasses when outdoors can help.

Popular story: Blog: 10 eyecare apps for more efficient patient care
Dr. Margie Recalde shares the top 10 mobile apps that can make an OD’s work life easier.
Learn her top apps!
GenSight Biologics reports positive 96-week data from phase III trial for LHON
GenSight Biologics reported a first set of results from Week 96 of the Reverse Phase III clinical trial. The trial evaluated the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 37 subjects whose visual loss due to 11778-ND4 Leber’s hereditary optic neuropathy (LHON) commenced between six and 12 months prior to study treatment.
Week 96 is the last of the scheduled readouts for the trial and marks the time when the data are unmasked, providing access to individual patient profiles.
The results point to continued efficacy of GS010 two years past injection, with best-corrected visual acuity (BCVA) sustaining a clinically meaningful improvement over baseline, according to the company.
At Week 96, GS010-treated eyes showed a mean improvement of -0.308 LogMAR compared to baseline, equivalent to +15.4 Early Treatment Diabetic Retinopathy Study (ETDRS) letters or three lines on the ETDRS vision chart. This level of improvement in visual acuity maintained the gain observed at Week 72 (+14.7 ETDRS letters equivalent).
Subjects with GS010-treated eyes had gained +28 more letters relative to their nadir, while low-contrast visual acuity-as measured on the Pelli-Robson contrast sensitivity chart-showed a similar trend of improvement for both GS010-treated eyes and sham-treated eyes.
The trajectories, however, did not track each other as closely as with BCVA. Mean contrast sensitivity for GS010-treated eyes showed more improvement versus baseline over the course of the trial.
The drop in the mean contrast sensitivity of sham-treated eyes, however, was not enough to yield a statistically significant difference in Week 96 mean contrast sensitivity improvement between the two sets of eyes.
Structural metrics indicate that GS010-treated eyes maintained the stability achieved in previous readouts in ganglion cell volume, but the differential effect of therapy was more prominent in previous readouts.

Popular story: How private equity affects optometry
The rise in private equity in optometry has changed the playing field for ODs looking for an exit strategy as well as those interested in purchasing a practice.
Learn how.
ABB Optical Group’s Glimpse Live debuts smartphone app to track business analytics
ABB Optical Group announced that its informatics service, Glimpse Live, has released a smartphone app for eyecare practitioners to track data and metrics of their practices.
Available as native iOS and Android apps, Glimpse Mobile features an interface with state-of-the-art data encryption.
The software is designed to help eyecare business owners measure and track their performance, identify growth opportunities, forecast future trends, and compare results among peers.
Glimpse Mobile complements Glimpse’s existing desktop application, which integrates with electronic health records (EHRs) and practice management systems to provide analytics; live benchmarking; employee productivity tracking; monthly strength, weaknesses, opportunities, and threats (SWOT) reports; and daily metrics in a snapshot format.
Popular story: Blog: Understand the science and art of managing chronic conditions
ODs play a key role in diagnosing and managing ocular chronic conditions. Find out why an understanding of these responsibilities can help provide better patient care.
Find out!
Transitions partners with Refinery29 for experiential tour
Transitions Optical is partnering with Refinery29 on its annual North American tour: 29Rooms: Expand Your Reality . The company will be providing industry partners the opportunity to attend the multi-city event.
The experience is expected to showcase the next generation of Transitions lenses, Transitions Signature GEN 8, which will be released in July.
The new photochromic lens technology is the result of five years of research and development involving more than 100 people.
Transitions Signature GEN 8 lenses are photochromic lenses. A new multi-criteria methodology was developed to assess the overall performance of photochromic lenses. This methodology is based on market research done in the U.S. among 1,000 eyeglass wearers.
Refinery29 has announced it is taking its 29Rooms annual immersive festival of style, culture, and creativity on a five-city North American tour, including its first international showing, in partnership with IMG.
The 2019 tour will complement 29Rooms’ marquee events in New York and Los Angeles, which have had more than 100,000 visitors from around the world since the event series first launched in 2015.
Each room in the Expand Your Reality experience will feature hands-on and phone-free activities. City-specific collaborations with local artists from Chicago, Dallas, Atlanta, Toronto, and Washington, D.C. will also be presented during the tour.
2019 tour locations and dates include:
• Chicago, Skylight Board of Trade: July 18-28
• Dallas, Gilley’s Southside Ballroom: Aug. 9-18
• Atlanta, The Works: Aug. 29-Sept. 8
• Toronto, Queen Elizabeth Building Exhibition Place: Sept. 26-Oct. 6
• Washington, D.C., D.C. Armory: Oct. 18-27
This week in optometry news: May 20, 2019
FDA approves Regeneron’s Eylea for diabetic retinopathy
Regeneron Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved Eylea (aflibercept) injection to treat all stages of diabetic retinopathy (DR).
The FDA approval of Eylea as a treatment for DR was based on six-month and one-year results from Panorama, a randomized, multi-center, controlled Phase III trial that enrolled 402 patients. The trial was designed to investigate Eylea for the improvement of moderately severe to severe non-proliferative DR (NPDR) without diabetic macular edema (DME), compared to sham injection.
“The prevention of worsening (DR) with Eylea provides a compelling rationale for early treatment of patients with this disease, says David Brown, MD, FACS, an investigator for the Panorama trial and director of Research at Retina Consultants of Houston, “particularly since eyes dosed with Eylea as infrequently as every 16 weeks showed significant improvements in the pivotal Panorama trial.”
The Panorama trial showed that by one year, 20 percent of untreated patients developed proliferative diabetic eye disease, according to George D. Yancopoulos, MD, PhD, president, and chief scientific officer at Regeneron.
“Eylea reduced this risk by 85 percent to 88 percent when administered using an every 16-week or eight-week dosing regimen, respectively,” Dr. Yancopoulos says. “In fact, 80 percent of patients who received the Eylea eight-week dosing regimen had significant improvement in their diabetic retinopathy.”
Eylea is the only vascular endothelial growth factor (VEGF) inhibitor approved with two dosing options for DR, allowing doctors to customize treatment to their patients' needs. In DR, Eylea may be dosed every eight weeks following five initial monthly injections, or every four weeks.
Panorama was the first prospective trial to study whether an anti-VEGF can also help prevent worsening disease in patients with NPDR without DME.

This week’s most popular story: How to control myopia progression in your practice
As the number of myopic patients increases across the globe, ODs need to know how to diagnose and slow the progression of myopia. Pamela Lowe, OD, FAAO, shares how she handles cases in her practice.
Find out!
EsssilorLuxottica, Delfin sign settlement agreement
EssilorLuxottica announced on May 13 that EssilorLuxottica and Delfin S.Ã .r.l. agreed upon a settlement agreement to overcome the governance challenges and set the basis for renewed collaboration between Essilor and Luxottica. The agreement settles any existing dispute among the parties.
EssilorLuxottica’s Board of Directors unanimously approved the agreement aimed at immediately making the group’s structure more efficient and effective from an operational standpoint.
The equal-powers governance, set forth in the Combination Agreement and the Board Rules, is remaining in place until the shareholders’ general meeting-to be called in 2021-to approve the financial statements for the year ended Dec. 31, 2020.
According to the agreement:
• Leonardo Del Vecchio and Hubert Sagnières asked Francesco Milleri (deputy chairman/CEO of Luxottica Group) and Laurent Vacherot (CEO of Essilor International) to develop and implement the EssilorLuxottica strategy and integration process, and integrate the two companies within the next 12 to 24 months.
• Milleri and Vacherot approved the appointment of key executives for the group’s central functions.
• Vacherot has been appointed as a director of EssilorLuxottica, replacing Bernard Hours, who asked to be relieved of his office. He will also become a member of the board's strategy committee.
• The board confirmed the search for a new CEO. Milleri and Vacherot have informed board members that they are not candidates for this position.
As a result of this agreement, all existing claims will be waived and legal proceedings terminated, including the request for arbitration filed by Delfin before the International Court of Arbitration of the International Chamber of Commerce on March 27.
In light of this agreement, Valoptec withdrew the proposal submitted on April 18 for the appointment of one additional director of EssilorLuxottica and will vote against the proposals submitted by institutional investors for the appointment of two additional directors.

Top-read story: Blog: Put profit first in your practice
Mick Kling, OD, shares why redefining and reevaluating your profits as a business owner are key to operating a successful business.
Read more here
Allegro Opthalmics announces positive dry eye trial results
Allegro Ophthalmics, LLC announced positive results of an ex-U.S. proof-of-concept clinical trial of investigational ALG-1007 topical drop drug candidate in patients with dry eye disease (DED).
The trial concluded that ALG-1007 demonstrated a dose response, indicating that the active pharmaceutical ingredient (API) in ALG-1007 is effective in improving the signs and symptoms of DED in as early as two weeks.
At the highest dose concentration (0.6%), ALG-1007 demonstrated statistically significant efficacy in nearly all assessments and a more rapid onset of action compared to the lowest dose (0.125%).
ALG-1007 was well-tolerated with no drug-related adverse events-even at the highest dose-and there was no reported blurring of vision or ocular irritation-including at the time of application, according to the company.
“Allegro has initiated a second and larger double-masked, vehicle-controlled ex-U.S. Phase II clinical trial, results of which are anticipated in the second half of 2019,” says Vicken Karageozian, MD, president and CEO of Allegro Ophthalmics.
The completed prospective, open-label, ex-U.S. proof-of-concept clinical trial enrolled 40 eyes of 21 patients diagnosed with DED for at least six months. Patients were assigned to one of four treatment doses (n=10, each group): 0.125%, 0.25%, 0.4% and 0.6% of ALG-1007 in a lubricating ophthalmic topical solution.
Topical treatment was administered as one drop twice a day, and subjects were followed at multiple timepoints for 12 weeks. Outcome measures were tear break-up time (TBUT), Sicca total ocular staining score, corneal and nasal conjunctival staining score, and reported symptoms using the visual analog scale (VAS) symptom index.
Popular story: Spectacles offer relief for headaches, ocular symptoms
Measuring binocular misalignment and prescribing a new spectacle lens with contoured prism may help your patients with persistent headache and ocular symptoms brought on by digital device usage.
Find out how!
McDougall Communications receives Bronze Anvil honors for CooperVision, CORE initiatives
The Public Relations Society of America has recognized two eye health programs spearheaded by McDougall Communications with 2019 Bronze Anvil honors.
CooperVision received a Bronze Anvil Award in the Research / Evaluation category for Eye Opening Insights Spark Optometrists to Act. The work brought together data and insights from eyecare professionals and consumers in the U.S., Europe, and Asia surrounding one-day silicone hydrogel contact lenses.

The Centre for Ocular Research and Education (CORE) received a Bronze Anvil Award of Commendation in the new digital platform category for CORE Knowledge: The Ultimate Eye Science Game.
Premiering at ARVO 2018, this tablet-based quiz presented more than 14,600 questions to optometry and ophthalmology attendees, representing approximately 1,100 games played on site. The quiz was co-developed with Rochester-based Workinman Interactive.
In all, 29 Bronze Anvil winners and 54 Award of Commendation winners across 33 categories were selected from more than 500 nominations.
“It’s hard to express how much we enjoy collaborating with CooperVision and CORE, working alongside their in-house marketing and clinical teams on a range of programs around the world,” says Mike McDougall, APR, Fellow PRSA, and president of McDougall Communications. “The 2019 Bronze Anvil honors also speak to the innovation and creativity happening within eye care.”

Popular story: Blog: A case of demodex infestation with eyelash extensions
Patients with eyelash extensions face an increased risk for developing demodex. Jade Coats, OD, explains why correctly identifying this infestation, as well as proper patient education on treatment and management, are keys to a successful outcome.
Learn more!
Genetic Disease Investigators receives new patent for dry eye treatment
Genetic Disease Investigators, LLC announced receipt of its second patent-U.S. Patent No. 10,238,673 B2-addressing dry eye treatment via the autonomic nervous system.
This patent is in addition to the company’s first patent, U.S. Patent No. 9,271,953, for the treatment of autonomic dysfunction.
The invention patents the treatment of dry eyes by targeting the parasympathetic nervous system, which is responsible for both tear production and the control of inflammation.
This dry eye therapy approaches dry eye treatment in two ways:
• Supports the lacrimal nerve by supplying everything the nerve needs to communicate with the lacrimal gland, which is responsible for basal tear production
• Supports the vagus nerve (also by supporting acetylcholine needed by this nerve) to help control inflammation that can cause or worsen dry eyes
All ingredients are generally regarded as safe (GRAS) by the Food and Drug Administration (FDA). These ingredients are combined into a supplement blend and are available without a prescription.

Popular story: Blog: 10 eyecare apps for more efficient patient care
Dr. Margie Recalde shares the top 10 mobile apps that can make an OD’s work life easier.
Learn her top apps!
DrChrono release new ‘Chart by Voice’ EHR feature for hands-free charting
DrChrono Inc announced the availability of Chart by Voice for the iPad EHR app. This new feature provides voice command for hands-free charting.
DrChrono’s Chart by Voice speech-to-text module enables clinicians to maneuver through a patient’s chart and quickly transcribe clinical notes by voice command in the EHR. The feature provides a more streamlined experience for patient encounters as well as faster charting.
For example, clinicians can now use voice commands such as “next field” to switch to the next field or say, “next paragraph” to add a line break instead of manually tapping on the screen. Clinicians can ask, “What can I say?” in the EHR app, and a glossary will appear with all voice commands available.
---
In other news, DrChrono and HealthFeed have partnered to give providers access to more educational content within the DrChrono EHR platform.
As part of DrChrono’s EHR platform, providers can now use the HealthFeed module to supply patients with medically validated health education illustrations and articles within the mediums they use the most, such as emails and text messages.
The module also includes video and Spanish content, and uses simple language patients can read and understand and will be able to access.
For providers, the HealthFeed module is a one-stop-shop to add various medically validated patient education content libraries, enhance current patient education programs, and save clinical time identifying, administering and sending education content to patients.
This week in optometry news: May 13, 2019
Novartis acquires Xiidra in $3.4 billion deal with Takeda
Novartis announced that it has entered into an agreement with Takeda Pharmaceutical Company Limited to acquire the assets associated with Xiidra (lifitegrast ophthalmic solution) 5% worldwide.
Xiidra is the first and only prescription treatment approved to treat both signs and symptoms of dry eye by inhibiting inflammation caused by the disease.
Closing of the transaction is expected in the second half of 2019, subject to customary closing conditions (such as regulatory approvals). On closing, Novartis plans to integrate Xiidra into its pharmaceuticals portfolio.
The additional commercial experience established with Xiidra is expected to better position Novartis for front-of-the-eye pipeline products currently in development.
Deal terms include a $3.4 billion upfront payment with potential milestone payments of up to $1.9 billion. As part of the agreement, Novartis will be taking on approximately 400 employees associated with the product.

This week’s most popular story: Blog: A case of demodex infestation
Patients with eyelash extensions face an increased risk for developing demodex. Jade Coats, OD, explains why correctly identifying this infestation, as well as proper patient education on treatment and management, are keys to a successful outcome.
Find out more!
Myopia Awareness Week targets global awareness
This week, World Council of Optometry (WCO) and Brien Holden Vision Institute (BHVI) are collaborating to highlight Myopia Awareness Week (May 13-19).
Both organizations are advocating for action in caregivers to protect children’s vision and change the way ODs understand and treat myopia.

“We know that almost half the world’s population will be myopic by the year 2050, with nearly 1 billion people in the high myopia category,” says Scott Mundle, OD, WCO president. “Myopia Awareness Week is about getting people talking about myopia in homes and optometry practices around the world.”
WCO and BHVI are addressing the prevalence of myopia globally by building an awareness that will help mitigate the effect myopia will have on billions of lives.
“We want caregivers to understand that children’s eye health is an important part of their overall health and development. We also want to make sure that ODs are equipped with the knowledge to help treat their myopic patients,” Dr. Mundle says.
WCO and BHVI aim to build a movement to fight the myopia epidemic that everyone sees coming, according to Dr. Mundle.
“There is much happening in research, product development, and professional education to meet the myopia challenge, but it is critical we engage with those at the frontline- parents, guardians and eyecare practitioners-to ensure they have the understanding and tools to protect our children’s futures,” says Yvette Waddell, CEO of BHVI. “This is an important and timely initiative involving the optometry profession globally.”
For more information about Myopia Awareness Week visit www.myopiamovement.com or visit social media accounts @MyopiaMovement.
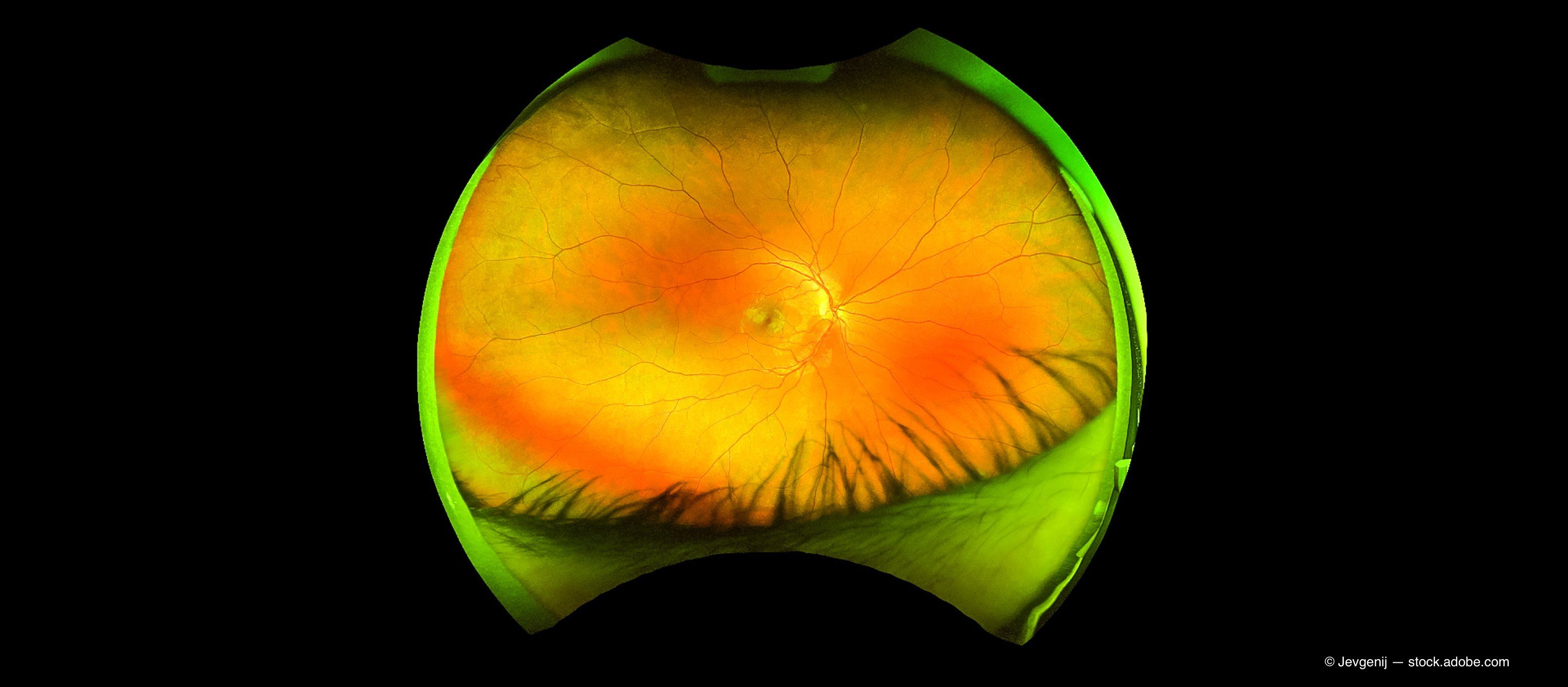
Learn why incorporating blood pressure measurements when performing glaucoma evaluations can be a quick and noninvasive way to keep an eye on potential lurking variables.
Read more here
Ellex launches new ultrasound technology
Ellex Medical Lasers announced the market launch of Eye Prime diagnostic ultrasound technology for evaluating pathology of the eye. Food and Drug Administration (FDA)-clearance was received on April 12.
The proprietary diagnostic advance features a multifocal array of six focal points to provide eyecare professionals with crisp focus to improve image definition and clarity.
The increased depth of field as well as real-time dynamic imaging permits materially enhanced viewing inside the eye. These structures cannot be seen with currently available competitive offerings, according to the company.

Popular story: Blog: Why medical coding is a big deal for ODs
John Rumpakis, OD, MBA, explains why it’s crucial for ODs to use the most specific and accurate medical codes, and how coding can affect a patient’s health records.
Find out!
Sylentis presents new drug for retinal diseases, DED treatment results
Sylentis presented the results of the preclinical development of its new compound, SYL1801, administered in eye drops for the prevention, treatment, and control of the progression of retinal diseases characterized by neovascular processes.
The results were presented at the Annual Congress of the Association for Research in Vision and Ophthalmology (ARVO) in Vancouver earlier this month.
The efficacy studies performed on an animal model of laser-induced choroidal neovascularization showed that the reduction of Notch-regulated akyrin repeat protein (NRARP) expression in the retina by SYL1801-administered in drops-is associated with a regression of angiogenic lesions.
The regressions observed are equivalent to those observed in the group of animals treated with intravitreally injected anti-VEGF agents (current standard treatment for diseases of the retina).
In addition, toxicology studies conducted on globally accepted animal models support that, at the tested concentrations and exposure times, SYL1801 is local and systemically well tolerated.
In other news, Sylentis presented its results of the clinical trial Helix with tivanisiran for the treatment of dry eye disease (DED).
The safety and efficacy of tivanisiran eye drops were evaluated in a population of 289 patients with persistent signs and symptoms of moderate to severe DED.
Tivanisiran, a small interfering ribonucleic acid (siRNA), showed significant results in:
• Statistically significant improvement in both signs and symptoms of dry eye syndrome in patients with Sjögren’s syndrome (p<0.05)
• Reduction of central corneal damage in patients with dry eye disease (p<0.05)
Amniotic membrane is another tool to allow ODs to offer an additional therapeutic treatment options to their patients with ocular surface disease, including dry eye.
Learn more!
COA says restoring Medi-Cal eyeglasses will save lives
The California Optometric Association (COA) released the following statement on May 9 from President Ronald G. Seger OD, FAOO, in response to the state governor unveiling a revised 2019-2020 state budget:
“Gov. Gavin Newsom’s May budget revision is welcome news for millions of Californians who are counting on California to keep the life-saving promise of Medi-Cal’s eyeglass and vision aid benefit. Eye doctors welcome the governor’s recognition that the eyeglass benefit affords seniors, people with disabilities, and low-income people the ability to see clearly so they can read, drive, and work. California’s ODs know well that restoring the eyeglass benefit means connecting more Californians with care that saves lives through early detection of diabetes and other chronic disease."
Dr. Seger also says that as the diabetes epidemic explodes across state, COA looks forward to working with state administration and legislation to implement preventative measures that will directly improve patients’ lives and health.
Gov. Newsom’s original budget plan-unveiled in January-proposed a broad overhaul of health care: lowering prescription drug costs, expanding Obamacare so middle-class families can receive subsidies to buy insurance, and offering Medi-Cal coverage to undocumented immigrants up to age 26. The revised budget was submitted based on the latest economic forecasts.
The revised budget includes $162.3 billion for all health and humans services-an increase of $1.1 billion in general funding compared to the governor’s original budget.
As digital device usage increases, so does potential for ocular damage, especially for children.
Find out more.
GenSight Biologics optogenetics trial gets positive safety review
GenSight Biologics announced that the independent Data Safety Monitoring Board (DSMB) completed its first safety review of the ongoing PIONEER Phase I/II clinical trial of GS030 combining gene therapy and optogenetics for the treatment of retinitis pigmentosa.
PIONEER is a first-in-man, multi-center, open label dose-escalation study to evaluate the safety and tolerability of GS030 in 18 subjects with retinitis pigmentosa. GS030 combines a gene therapy (GS030-DP) administered via a single intravitreal injection with a wearable optronic visual stimulation device (GS030-MD).
GS030 is based on the optogenetics technology platform developed by GenSight, which uses gene therapy to introduce a gene encoding for a light-sensitive protein into retinal ganglion cells by a single intravitreal injection, making them responsive to light and bypassing disease-destroyed photoreceptors.
DSMB confirmed the absence of safety concerns for the first cohort of three subjects who received a single intravitreal injection of 5e10 vg combined with a wearable optronic visual stimulation device.
The board recommended moving forward as planned without any modification in the protocol as well as recruiting the second cohort of three subjects receiving an escalating dose of 1.5e11 vg.
Eligible patients in the first three cohorts will be those affected by end-stage nonsyndromic retinitis pigmentosa with no light perception or light perception levels of visual acuity. The extension cohort will include patients with hand motion and counting fingers levels of visual acuity.
The primary outcome analysis will be the safety and tolerability at one-year post-injection.
GenSight expects to complete enrollment in the first half of 2020. Early findings may be available and released in the second half of 2019, and preliminary results are expected in the fourth quarter of 2020.
This week in optometry news: May 6, 2019
Sight Sciences launches TearCare, enhanced Omni Surgical System
Sight Sciences, Inc. announced the official commercial launch of TearCare and the introduction of an aesthetically and ergonomically enhanced OMNI Surgical System (which was initially launched in February 2018).
“We have built a highly robust and pedigreed commercial force comprised of two discrete, specialized sales and marketing teams for both the dry eye (TearCare) and minimally invasive surgery (MIGS) (Omni) practice areas," says Shawn O’Neil, chief commercial officer (CFO) of Sight Sciences. “We are well-positioned to further accelerate the market adoption of Omni in the coming months as we roll out our next generation product and to execute a best-in-class commercial launch for TearCare.”

This week’s most popular story: Blog: A case of demodex infestation
Patients with eyelash extensions face an increased risk for developing demodex. Jade Coats, OD, explains why correctly identifying this infestation, as well as proper patient education on treatment and management, are keys to a successful outcome.
Find out more!
Bausch + Lomb announces update from antibiotic resistance monitoring in ARMOR study
Bausch + Lomb announced the results from nearly 10 years of the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study, presented at the 2019 Association for Research in Vision and Ophthalmology (ARVO) meeting in Vancouver.
Researchers also presented preliminary 2018 surveillance data on antibiotic resistance levels. Initiated in 2009, ARMOR is the only ongoing multicenter survey of antibiotic resistance patterns specific to ocular pathogens in the U.S.
The ARMOR study tracks in vitro antibiotic susceptibility profiles among ocular bacterial pathogens of significance. Clinically relevant isolates of Staphylococcus aureus (S. aureus) coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae from ocular infections are collected and subjected to antibiotic susceptibility testing.
In the longitudinal trend analysis presentation, ARMOR researchers reported resistance trends in staphylococcal infections, which included 2108 S. aureus and 1721 CoNS that were collected since January 2009.
The results of the analysis confirmed the previously reported decrease in methicillin resistance (MR) among S. aureus (P<0.001; 40 percent in 2018), but not among CoNS (P=0.762), with approximately half of CoNS exhibiting MR each year.
In the 2018 preliminary update, ARMOR researchers reported a total of 414 isolates collected from 15 participating sites in the U.S. Among staphylococci, resistance rates appeared similar to 2017 rates, with considerable resistance among staphylococci to azithromycin (52 to 60 percent), oxacillin/methicillin (30 to 49 percent), and ciprofloxacin (30 to 31 percent).
CoNS isolates also exhibited resistance to trimethoprim (25 percent) and tobramycin (23 percent). Multidrug resistance (MDR) was observed in 30 percent of S. aureus and 40 percent of CoNS and was especially prevalent among methicillin-resistant staphylococci (72 to 77 percent). Isolates of S. pneumoniae were resistant to azithromycin (33 percent) and oral penicillin (28 percent) with no resistance to fluoroquinolones.
All isolates of P. aeruginosa were susceptible to fluoroquinolones with 6 percent exhibiting resistance to polymyxin B. With the exception of a single tetracycline-resistant isolate, all H. influenzae were susceptible to tested drugs.

Top-read story: What happened in Oklahoma: Expanding scope of practice and protecting what has been earned
In the latest Defocus Media podcast, Jason Ellen, OD, of Oklahoma discusses the recent state legislative battle ODs fought to defend their rights to practice to the full scope as outlined by Oklahoma law.
Listen and read here!
Uncorrected myopia cost global economy U.S. $244 billion in lost productivity in 2015
Vision impairment caused by uncorrected myopia cost the global economy an estimated U.S. $244 billion in lost productivity in 2015, according to a new study published in Ophthalmology.
The research estimated that 538 million people had vision impairment resulting from uncorrected myopia, with the East Asia region-which includes China-bearing the greatest burden of productivity loss of around U.S. $150 billion.

The South Asia and South East Asia regions also experienced significant productivity loss at over U.S. $30 billion each. This represents in excess of 1 percent of gross domestic profit (GDP) in each of the three regions.
The authors say a one-off investment of about U.S. $20 billion would establish the services necessary to provide vision correction to all who need it, potentially leading to a significant annual saving in productivity.
A combination of factors explains the substantial burden in East Asia, according to Padmaja Sankaridurg, professor and head of Myopia at Brien Holden Vision Institute.
“The high-density urban living with a focus on near based activities has resulted in high prevalence and also in a large number of people with inadequate visual correction,” she says.
Along with East Asia, other regions significantly impacted are South Asia and Southeast Asia, both estimated to suffer productivity loss of over U.S. $30 billion in 2015.
The researchers conclude that people with myopia are less likely to have adequate optical correction if they are older and live in a rural area of a less developed country.
The researchers noted that lost productivity resulting from myopia-related vision impairment represents only part of the overall economic burden of myopia. Direct costs such as expenses related to eye examinations, refractive corrections and managing pathological consequences of myopia such as myopic macular degeneration (MMD), and related opportunity costs, are not covered in their analysis.
The study "Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis and modelling" was conducted by researchers from:
• Brien Holden Vision Institute
• University of New South Wales (Australia)
• African Vision Research Institute
• University of KwaZulu Natal (South Africa)
• Carey Business School at Johns Hopkins University (U.S.)
Research was supported with funding from Brien Holden Vision Institute and the Vision Impact Institute.
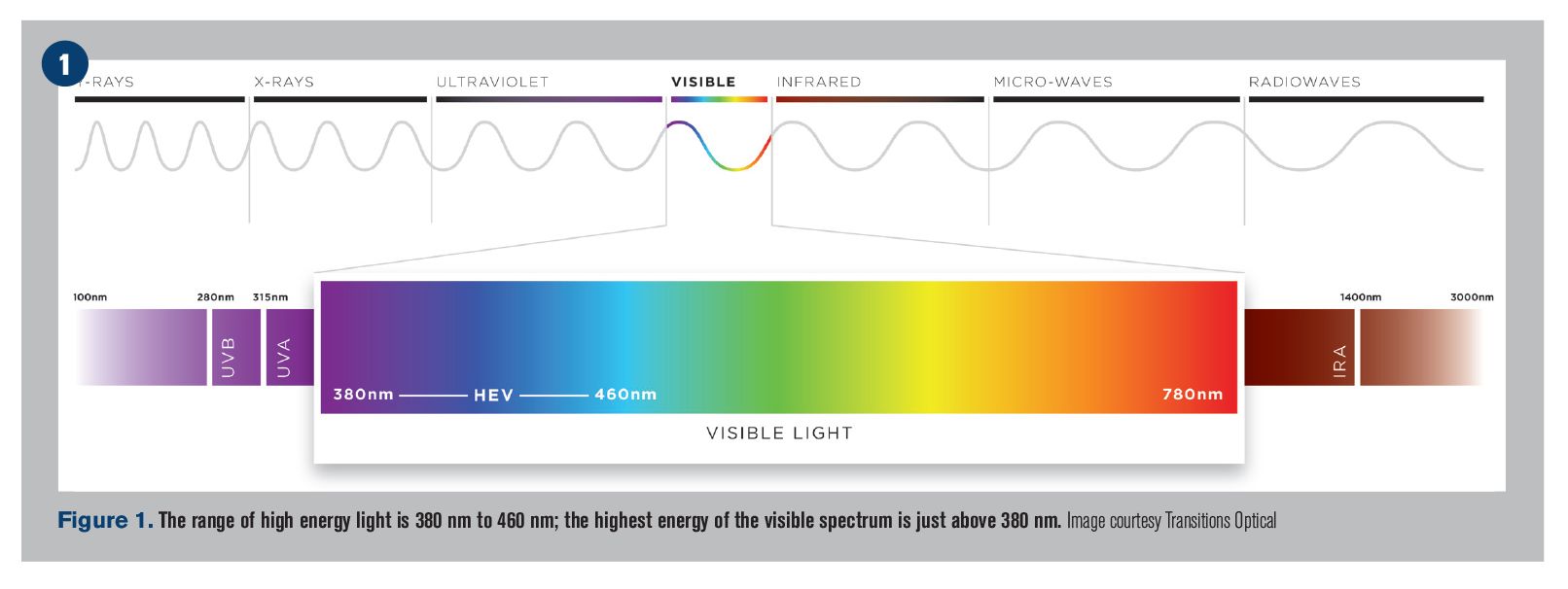
Popular story: Blue light: Why it matters
As digital device usage increases, so does potential for ocular damage, especially for children.
Find out more.
Submission window opens for Academy 2019 Orlando, 3rd World Congress of Optometry
The Scientific Program Committee invites the submission of abstracts for Academy 2019 Orlando and 3rd World Congress of Optometry, to be held Oct. 23 through Oct. 27.
The Scientific Program offers researchers, educators, and clinicians the opportunity to exchange the latest information in optometry and vision science in two formats, research paper presentations and scientific posters.

The abstract submission window is open May 1 through May 31. This year, the Scientific Program Committee will organize multiple focused paper and poster sessions on special topics that will offer attendees an opportunity to engage in lively discussions and debates.
The Scientific Program Committee will consider all presentations, including those from students and residents. Submissions of previously published copyrighted material (such as ARVO abstracts or journal articles) will not be accepted. Abstracts will be judged on the following criteria:
Research abstracts
• Level of scientific or clinical novelty
• Methodological soundness
• Quantitative description of results
• Statistical analysis for significance of results
• Support of conclusions by results
• Adherence to submission instructions and ethical standards
• Language and grammar
Case reports
• Level of clinical novelty
• Completeness of case work-up to support proposed diagnosis
• Sufficient author involvement in the examination, diagnosis and/or management of case
• Adherence to submission instructions and ethical standards
• Language and grammar
First authors (excluding students and residents) of accepted papers/posters are also eligible to register for the meeting at reduced rates.

Popular story: Troubleshoot contact lens discomfort and prevent complications
Ocular surface disease can be further complicated by contact lens wear. Keep an open mind to the possible underlying conditions, even with asymptomatic patients.
Learn more.
Aerie announces U.S. launch of Rocklatan, increase in Rhopressa Medicare Part D coverage
Aerie Pharmaceuticals, Inc., announced that it has launched Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% into the U.S.
glaucoma market. Rocklatan is now with national and regional U.S. pharmaceutical wholesalers, and patients can fill prescriptions for Rocklatan through their local pharmacies across the nation.
Additionally, Medicare Part D preferred tier coverage for Rhopressa (netarsudil ophthalmic solution) 0.02% has increased from approximately 40 percent to 75 percent. Commercial coverage for Rhopressa remains at 90 percent of lives, with 55 percent of lives in a preferred tier.
“We are also providing a single savings card to reduce out-of-pocket costs for commercially-insured patients who are prescribed either Rocklatan or Rhopressa,” says Vicente Anido, Jr., PhD, chairman and CFO. “Ultimately, we believe Rocklatan has the potential to become a new cornerstone of medical therapy for glaucoma and ocular hypertension.”

Popular story: How to diagnose a swollen optic nerve
There is not much more intimidating for an OD than for a patient to come in complaining of vision loss and the dilated fundus exams reveals a swollen optic nerve(s).
Read on!
Abeona Therapeutics reports preclinical data supporting potential of AIM gene therapy in retinal diseases
Abeona Therapeutics Inc. announced that intravitreal administration of the company’s novel AIM AAV204 capsid to non-human primates led to robust transgene expression in the inner and outer retina.
These new preclinical data also support the potential use of intravitreal administration to deliver gene therapy in an out-patient setting for a wide range of inherited and acquired retinal diseases.
Findings were presented at ARVO annual meeting in Vancouver.

In the preclinical study, intravitreal administration of the novel AIM AAV204 capsid in non-human primates resulted in broad transgene expression in the peripheral retina, as well as intense expression in the fovea 25 days post-administration.
AAV204 also transduced photoreceptor cells in retinal explants and transduced the outer retina, with positive green fluorescent protein (GFP) expression.
The non-human primate data were complemented by findings from mice models, which identified AAV204 as one of three lead candidate AIM capsids that demonstrate robust transduction of retinal cells.
The data in mice demonstrated that intravitreal administration resulted in broad retinal expression of AAV204 that penetrated to the photoreceptor and retinal pigmented epithelium (RPE) layers.

Popular story: How to educate patients on risks of eyelash enhancements
Fake eyelashes are a growing beauty trend among makeup consumers across the globe. But ocular surface complications can arise when proper management and treatment are not practiced. Educate patients on recognizing these signs and how to avoid them.
Learn how.
Avedro completes enrollment in Phase II of epi-on corneal CXL trial for progressive keratoconus
Avedro, Inc. announced that the company has completed patient enrollment in a pivotal Phase 3 clinical trial to evaluate the safety and efficacy of an epithelium-on (epi-on) corneal cross-linking (CXL) procedure for the treatment of progressive keratoconus.
If approved, the company anticipates that its product offering would be the first FDA-approved non-invasive CXL procedure that does not require removal of the epithelium.
The Phase 3 clinical trial, ACP-KXL-308, is a multicenter, randomized, sham-controlled study of a novel CXL procedure that includes Avedro’s latest-generation UV light source, supplemental oxygen designed to enhance cross-linking, and a new drug formulation designed to penetrate the epithelium.
The study has enrolled patients across 14 centers in the U.S., with more than 275 eyes included.
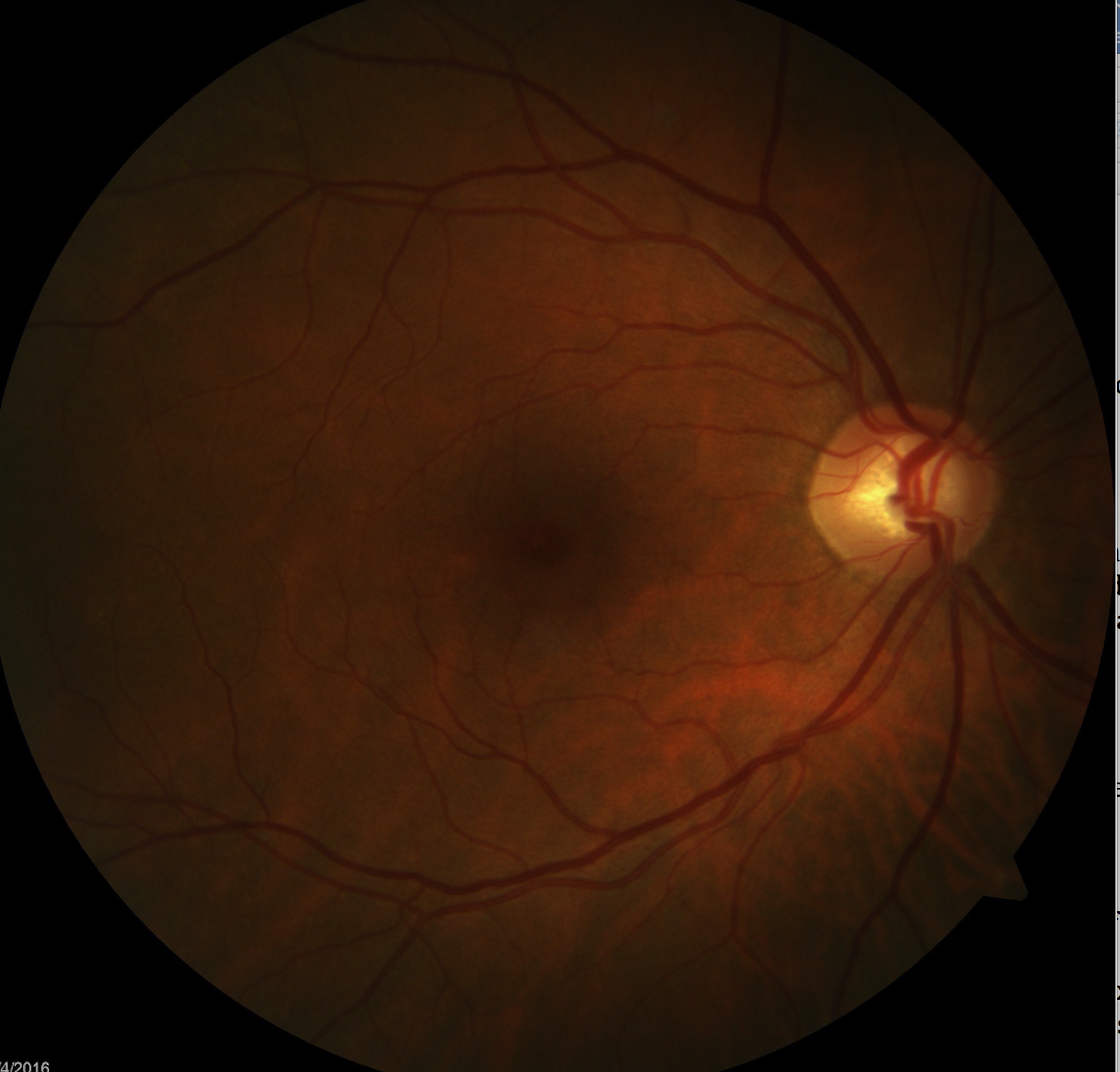
Popular story: Know the link between cotton wool spot and anemia
A cotton wool spot found during an annual comprehensive exam on an otherwise healthy 54-year-old female may have lead to a life-saving diagnosis.
Find out.
Sydnexis enrolls first patients in Phase III myopia study
Sydnexis Inc. announced that the first patients have been successfully dosed in a Phase III multi-center trial of SYD-101 following FDA clearance in April.
Sydnexis’ Safety and Efficacy of SYD-101 in Children with Myopia (STAAR) Study will be the largest myopia study initiated to date with more than 800 patients.
In a placebo-controlled trial, the STAAR study will test the safety and efficacy of two dosage strengths of SYD-101, the company’s patented investigational drug (a formulation of atropine), as a first-line therapy to slow the rate of myopic progression in children.
SYD-101 was designed for maximum stability and tolerability and will be dosed nightly as a single drop in each eye.
Sydnexis is actively enrolling patients at multiple sites.

Popular story: How I handled an unusual contact lens request
An OD received an unusual request about contact lenses for a climbing trip. Would you have responded the same way?
Find out!
Lighthouse Guild: integrating vision, healthcare services essential for older adults
Longer lifespan has increased the number of individuals living with vision loss from age-related diseases. As vision loss is increasingly a problem of aging, effective health care must address the social, environmental, and behavioral aspects of functional behavior and wellbeing, according to a presentation by Alan R. Morse, JD, PhD, president and CEO of Lighthouse Guild, at the ARVO Annual Meeting in Vancouver.
“Because people living with vision loss most often have other health issues, all service providers, including primary-care physicians, dentists, podiatrists, physical therapists, and internists have to understand their roles in identifying, providing care for, and referring patients for specialized services,” Dr. Morse says.
He says that the onset of legal blindness increases the likelihood of limitations of activities of daily living. He also noted that as many as 30 percent of patients with vision impairment have depression, a condition which has its own decompensating trajectory.
In hospitals, vision loss is rarely addressed in the patient's care plan-even though patients with vision loss stay in the hospital longer, experience more problems after discharge, and, overall, are less satisfied with their health care.
Dr. Morse called for a greater understanding of the importance of vision loss to everyday functioning and wellbeing to better meet patient needs.
Popular story: How to teach skills for successful technicians
Sharon M. Brown, COE, COT, and Lisa M. Miller, COMT, COE, explains why establishing a comprehensive training program pays dividends now and down the road.
Read on!
Comprehensive new HR support added for IDOC members
IDOC has announced the launch of the latest enhancement to IDOC HR Services that will help to streamline and standardize the process of hiring, developing, and managing qualified practice staff members, according to the company.
Available to IDOC members, the broad-based human resources support is customized for the needs of independent optometrists.

The newly designed IDOC HR platform includes:
IDOC employee testing service
An assessment tool that provides side-by-side comparisons of prospective employees during the hiring process, along with personalized onboarding and development strategies.
The service is available to all IDOC members for a per-use fee. It can also be used for existing employees.
IDOC employee handbook service
This service provides a practice handbook with customized guidelines for workplace interactions, performance evaluation, and benefit packages. It may help both ODs and staff members comply fully with changing workplace rules and regulations in every state.
Access to IDOC employee handbook service is available to all IDOC members and non-members for a fee.
This week in optometry news: April 29, 2019
Bausch + Lomb recycles over 9.2 million units used contact lenses

Bausch + Lomb announced on Earth Day that its One by One Recycling Program, the first contact lens recycling program of its kind, has recycled more than 9.2 million used contact lenses, blister packs and top foils since the program’s launch in November 2016.
The recycling program is offered free of charge to eyecare professionals and their patients across the U.S., and is made possible through a collaboration with TerraCycle, a world leader in the collection and repurposing of hard-to-recycle post-consumer waste.
As of the end of March 2019, the total amount of waste collected and recycled translates to more than 55,200 pounds-roughly the weight of an adult whale shark.
According to The Association of Plastic Recyclers, the industry-standard screen size, which identifies and removes unrecyclable plastics, filters out materials that measure less than three inches in diameter. Because standard recycling facilities are unable to process these small items, they either end up contaminating other recyclable material or are diverted to landfills.
To participate in the program, contact lens wearers are encouraged to bring their used contact lenses and packaging to any of the more than 3,500 participating eyecare professionals’ offices and recycle them in custom recycling bins provided to registered accounts.
Once the recycling bins are full, the practice mails the used lens materials to TerraCycle for proper recycling using a free shipping label from www.bauschrecycles.com. Once the materials are received by TerraCycle, the materials are then recycled into post-consumer products.
For every qualifying shipment of waste that weighs ten pounds or more from a practice, a $1 per pound donation is made to Optometry Giving Sight, the only global fundraising initiative specifically targeting the prevention of blindness and impaired vision by providing eye exams and glasses to those in need.
To register and learn more about the Bausch + Lomb One by One Recycling Program, visit www.BauschRecycles.com.

This week’s most popular story: Blue light: Why it matters
As digital device usage increases, so does potential for ocular damage, especially for children.
Find out more.
Bausch + Lomb initiates series of U.S. clinical trials for technolas teneo excimer laser
Bausch + Lomb will initiate a series of U.S. clinical trials to evaluate the safety and efficacy of technolas teneo excimer laser for vision correction surgery for myopia and myopic astigmatism. The company expects the clinical trials to begin by July 2019.
The system being studied will be the same as the technolas teneo laser (model 2) currently being sold outside the U.S. Bausch + Lomb plans to file for premarket approval (PMA) of the technolas teneo laser from the U.S. Food & Drug Administration (FDA) at the conclusion of the clinical trials.

Top-read story: How to diagnose a swollen optic nerve
There is not much more intimidating for an OD than for a patient to come in complaining of vision loss and the dilated fundus exams reveals a swollen optic nerve(s).
Read on!
EssilorLuxottica Board of Directors vote against proposed resolutions
EssilorLuxottica’s Board of Directors met April 24 to review the proposed complementary resolutions submitted on April 18 by Valoptec and certain institutional investors, on opposing sides, for the appointment -by the May 16 general meeting -of, respectively, one additional board member and two additional board members.
The Board of Directors recommended that the shareholders vote against all proposed resolutions which, if approved, would result in a clear breach of the Combination Agreement and a potential disruption for the activities of the Board.
Allegro Ophthalmics expands anti-integrin portfolio with ALG-1007 for DED treatment
Allegro Ophthalmics, LLC, a privately held biopharmaceutical company focused on the development of novel anti-integrin therapies for the treatment of ocular diseases, today announced the expansion of the company’s anti-integrin portfolio with the new front-of-the-eye-drug candidate ALG-1007. ALG-1007 is a topical treatment in development for potential use in patients with dry eye disease (DED.
ALG-1007 bolsters Allegro’s existing anti-integrin portfolio, which includes risuteganib (Luminate), currently being developed for diabetic macular edema (DME) and intermediate dry age-related macular degeneration (intermediate dry AMD).
Pre-clinical and clinical findings of risuteganib convinced the company that it could develop a new anti-integrin compound to treat DED.
Allegro also announced that clinicians Richard L. Lindstrom, MD, Edward J. Holland, MD, and Eric D. Donnenfeld, MD, have joined the company’s newly formed Cornea Scientific Advisory Board (Cornea SAB) to provide strategy and direction on Allegro’s clinical development pipeline in the areas of corneal disease and dry eye. Dr. Lindstrom will serve as chairperson of the Cornea SAB.

Popular story: Keep an eye on link between glaucoma and blood pressure
Learn why incorporating blood pressure measurements when performing glaucoma evaluations can be a quick and noninvasive way to keep an eye on potential lurking variables.
Learn more!
Envision issues calls for submissions, registration for fall conference
Envision is now accepting clinical education and research presentation submissions through June 30 for Envision Conference West, the premier accredited multidisciplinary event focusing on low vision and low vision rehabilitation.

The conference will be held Oct. 5-6 at the College of Optometry at Western University of Health Sciences in Pomona, California. Both submissions and participant registrations can be made through the Envision Conference website, www.envisionconference.org.
An early bird online registration rate is available until July 31 for attendees who will gain access to the latest advances to close practice gaps, learn about developments in assistive technology, interact with researchers, hear crossover considerations in fields such as neurology and psychology, network with fellow practitioners from around the globe and earn continuing education credits.
The Envision Conference planning committee is currently seeking intermediate and advanced content, particularly presentations involving the following:
• Collaborations between low vision researchers, practitioners, educators, and healthcare organizations
• Co-management models of interdisciplinary care
• Pediatric vision loss and rehabilitation
• Neuro-visual deficits
• Applied research in assistive technology, clinical practice outcomes measures, and vocational accessibility and outcomes
• National and international exchanges of vision rehabilitation information among individuals, groups and institutions
• Patient communication and dual sensory loss
• Strengthening role of low vision on public health agenda
• Disparities in access to low vision care
• Practice gaps in low vision care delivery methods
• Addressing national eye health
Held twice annually at locations across the country, Envision Conference attracts ODs, ophthalmologists, researchers, occupational therapists, rehabilitation therapists, nurses, teachers of the visually impaired, special education teachers and others.
Sessions also offer the opportunity to earn continuing education credits from agencies including:
• Council on Optometric Practitioner Education (COPE)
• American Occupational Therapy Association (AOTA)
• Academy for Certification of Vision Rehabilitation & Education Professionals (ACVREP)
• Commission on Rehabilitation Counselor Certification (CRCC)
Complete details about Envision Conference, including a link to the submissions site, can be found online at www.envisionconference.org.
Popular story: Macular degneration A to Z
Get up-to-date knowledge on the most important concepts associated with the diagnosis and treatment of AMD.
Find out.
Visoneering Technologies, Inc. and Lambda-X develop software showing accurate power profile of NaturalVue MF contact lenses
Visioneering Technologies, Inc (VTI) has released additional data regarding its patented NaturalVue Multifocal (etafilcon A) one-day contact lens.
Scientific interest
Practitioners employing VTI’s NaturalVue Multifocal in managing myopia in children recently announced that 141 children wearing the contact lenses had a decreased progression of myopia by an average of 90 percent, compared to the average progression rate before wearing the contact lenses.
The treatment effect, which included up to four years of treatment with NaturalVue Multifocal, was consistent over time and across clinical sites.
The patent for the design of the NaturalVue Multifocal describes the optics of this extended depth-of-focus lens in terms of instantaneous power. While familiar to specialists, instantaneous power may be less familiar to the broader community. VTI is releasing additional information to assist in the understanding of the lens design.
The scientific community uses two methods of expressing the power profile in a lens design:
• Sagittal (also called axial or radial)
• Instantaneous (also called tangential)
Both methods are useful in creating and describing lens designs, but provide different information and are used for different purposes.
NaturalVue Multifocal contact lenses are progressive aspheric lenses best described in terms of instantaneous power, consistent with how VTI’s and engineers view progressive aspheric optical designs.
However, wavefront metrology typically describes power profiles in terms of sagittal power, and the conversions for calculating instantaneous power were lacking. VTI worked with Lambda-X-a global leader in ophthalmic products, including its NIMO instruments- to develop customized software capable of measuring and illustrating the instantaneous and sagittal power curves of the NaturalVue Multifocal, or any other multifocal lens.
The neurofocus optics technology of the NaturalVue Multifocal relies on the rapid, continuous, and uninterrupted progression in plus power to induce an aperture effect for clear vision at all distances.
Published data suggests that significant levels of plus power in the lens optical design is important to addressing peripheral defocus in the eye. The progression of plus power in the NaturalVue Multifocal design is significant enough to induce peripheral blur, generating a virtual aperture effect for an extended depth of focus.

Popular story: Blog: 10 eyecare apps for more efficient patient care
Dr. Margie Recalde shares the top 10 mobile apps that can make an OD’s work life easier.
Learn her top apps!
Visioneering Technologies, Inc creates educational series for eyecare practioners
Visioneering Technologies, Inc (VTI) has created a new educational series for eyecare practitioners featuring some of the world’s leading researchers and clinicians.
The first installment of the VTI / NaturalVue video series highlights the following speakers and topics:
• Earl Smith, professor and researcher, University of Houston, United States-Impact of eye growth, disease states
• Dr. Nicola Anstice, researcher, University of Canberra, Australia-Contact lenses and Safety with Children
• Padmaja Sankaridurg, professor and researcher, Brien Holden Vision Institute, Australia-Future prevalence and implications of myopia
• Dr. Kate Gifford, clinician-scientist and peer educator, Australia-Helping parents manage childhood myopia
• Dr. Sally Dillehay, clinical researcher and optometrist, (and former VTI employee), U.S.- Disease states and impact of genetics
The educational series is free for eyecare practitioners and can be accessed via: https://vtivision.uk/practitioners/educational-video-series/.

Popular story: Cataract surgery comanagement from the other side
One OD working in a surgery center shares how he and his colleageus comanage with referring ODs, and why they say this makes for better patient outcomes.
Read on!
Salus University awards medal of honor to university president
On April 26, 10 individuals were awarded the first-ever Salus University Presidential Medals of Honor to commemorate an important milestone: 10 years of the establishment of Salus University.
The award ceremony was part of a larger centennial celebration of the Pennsylvania Colelge of Optometry (PCO). The new recipients for the medals were nominated by the University’s Centennial Committee. Each recipient was chosen for their significant contributions to their profession and/or for their service to the institution.
Among those to receive the first-ever Salus University Presidential Medals of Honor was Michael H. Mittelman, OD, MPH, FAAO, FACHE, and Salus University president.

“The Salus Board is thrilled that Dr. Mittelman is being recognized for his achievements at PCO, Salus, as well as being an ‘ambassador’ for the school while serving in the Navy,” says Jo Surpin, Salus University board chair. “As president, he has provided the leadership necessary to continue the transition from PCO to Salus University while preserving our legacy.”
Dr. Michael H. Mittelman served for more than three decades in the U.S. Navy. He achieved the rank of rear admiral (upper half), and served as deputy surgeon general.
He served as the first Navy aerospace OD and first OD to command a major naval hospital; the first-and only-clinician to lead the Navy Medical Service Corps; the first non-medical doctor to serve as a combatant command surgeon in the U.S. Pacific Command; and the first non-medical doctor to serve as the command surgeon for the U.S. Joint Forces.
The full list of the awardees and their bios can be found on the university’s Centennial website: salus.edu/centennial.
The week in optometry news: April 22, 2019
Novartis announces FDA filing, priority review of brolucizumab (RTH258) for wet AMD
Novartis announced that the U.S. Food and Drug Administration (FDA) accepted the company's biologics license application (BLA) for brolucizumab (RTH258) for the treatment of wet age-related macular degeneration (AMD), also known as neovascular AMD or nAMD.
Seeking to make brolucizumab available as quickly as possible, Novartis used a priority review voucher to expedite FDA review. If approved by the FDA, Novartis anticipates launching brolucizumab by the end of 2019.
Estimates suggest that by 2020, 1.5 to 1.75 million people in the U.S. will be living with wet AMD, a rapidly growing public health concern.
The regulatory application is primarily based on Phase III data from the HAWK and HARRIER trials-prospective, randomized, double-masked multi-center studies. The primary endpoint of these studies was non-inferiority to aflibercept (Zaltrap, Sanofi) in mean change in best-corrected visual acuity (BCVA) from baseline to Week 48 (mean change in BCVA of 6.6 letters for brolucizumab 6 mg versus 6.8 letters for aflibercept in HAWK and 6.9 letters versus 7.6 letters, respectively, in HARRIER).
HAWK and HARRIER are the first and only global head-to-head trials in patients with wet AMD that prospectively demonstrated efficacy at Week 48 starting with a 12-week dosing regimen, according to the company.
Additionally, at Week 48 in the studies, key secondary endpoint assessments showed significantly fewer brolucizumab patients with disease activity (23.5 percent of brolucizumab 6 mg patients versus 33.5 percent of aflibercept patients in HAWK, and 21.9 percent versus 31.4 percent, respectively, in HARRIER (P=0.0022 for both) as well as retinal fluid.
These are key markers used by physicians to help guide management of the disease in clinical practice (31 percent fewer patients on brolucizumab 6 mg had intra-retinal fluid (IRF) and/or sub-retinal fluid (SRF) in HAWK, and 26 percent fewer in HARRIER, versus aflibercept (P<0.0001 for both).

This week’s most popular story: How to see 50 patients a day at your practice
Seeing a high volume of patients is more than just efficiency.
Find out!
EssilorLuxottica initiates CEO search with support of two agencies
In line with the agreement reached between Delfin and Essilor prior to the closing of the business combination between Essilor and Luxottica, the EssilorLuxottica Nomination and Compensation Committee has retained two recruitment agencies to assist in finding candidates to serve as chief executive officer and to be appointed by the end of 2020.
Pursuant to the existing agreements, the future CEO will be agreed and proposed to the Board of Directors for joint appointment by both the executive chairman and the executive vice-chairman, and recommended by the Nomination and Compensation Committee.
The selected candidate shall initially be appointed as “directeur général délégué,” and be in charge of coordinating the activities of EssilorLuxottica as a pure holding and assisting the executive chairman and the executive vice-chairman in their efforts to facilitate the integration of Essilor and Luxottica.
The new company’s powers shall be defined by the Board based on the joint recommendation of the executive chairman and the executive vice-chairman.
In the meantime, Luxottica and Essilor shall each remain separate legal entities fully empowered to run their own business autonomously and under their own leaderships and CEOs with certain exceptions.
The mandate was given to Russell Reynolds Associates and Eric Salmon & Partners and includes evaluations of both internal and external candidates. Russell Reynolds Associates will coordinate the process.
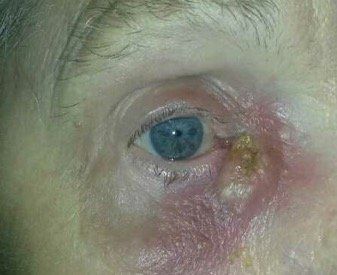
Top-read story: Blog: How I managed a day of ER referrals
One OD sees patients referred by the local hospital. It was interesting day.
See how!
NECO inaugurates Howard B. Purcell as president
New England College of Optometry (NECO) has inaugurated Howard B. Purcell, OD, FAAO, as president. Dr. Purcell is the 13th president in the college’s 125-year history. He is a 1984 graduate of the program, continuing the legacy of his father, Saul Purcell, OD’54.
The ceremony took place on April 7 at the Edward Kennedy Institute for the U.S. Senate in Boston in a recreation of the U.S. Senate chamber.
In his role, Dr. Purcell will address:
• Student applicant pipeline
• Research funding competition
• Lack of diversity in optometry
• Student debt
• Adequate care for at-risk populations
Dr. Purcell outlined his strategic priorities for the college to help “prepare today’s optometrists for tomorrow’s optometry.”
His vision includes:
• Diversifying revenue
• Nurturing diversity, equity and inclusion
• Expanding continuing education and specialized degree programs
• Collaborating with industry
• Finding ways to best support and prepare students
Dr. Purcell stepped into his role as president on July 1, 2018.

Popular story: Blog: 10 eyecare apps for more efficient patient care
Dr. Margie Recalde shares the top 10 mobile apps that can make an OD’s work life easier.
Learn her top apps!
GenSight Biologics reports positive results for GS010 in LHON
GenSight Biologics announced results from the second scheduled readout of the RESCUE Phase III clinical trial evaluating the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) on 39 subjects whose visual loss due to 11778-ND4 Leber hereditary optic neuropathy (LHON) occurred up to six months prior to study treatment.
These subjects received GS010 in one eye and a sham injection in the other eye, with drug treatment randomized between best- and worst-affected eyes.
The key measure of visual function-best-corrected visual acuity (BCVA)- continued to improve at Week 72 compared to Week 48, demonstrating sustained recovery from the lowest point, or nadir, experienced in the acute phase of the disease.
By Week 72, GS010-treated eyes improved by -0.413 LogMAR (+21 early treatment diabetic retinopathy study[ETDRS] letters equivalent) from nadir, compared to the Week 48 improvement of -0.257 LogMAR (+13 ETDRS letters equivalent).
This recovery at Week 72 could not yet completely offset deterioration from baseline through the acute phase: GS010-treated eyes were still below baseline by 0.192 LogMAR (-10 ETDRS letters equivalent), compared to 0.380 LogMAR (-19 ETDRS letters equivalent) at Week 48.
Consistent with all readouts so far in the RESCUE and REVERSE trials, sham-treated eyes had a BCVA evolution that closely tracked that of GS010-treated eyes.
At Week 72 of RESCUE, sham-treated eyes improved by -0.435 LogMAR from nadir (+21.7 ETDRS letters equivalent). The U-shaped curve thus closely matched that of GS010-treated eyes, so a statistically significant difference in visual acuity between GS010- and sham-treated eyes could not be shown.
The strength of the bilateral recovery shifted the mean BCVA in both sets of eyes from being off-chart at Week 48 to on-chart at Week 72.
Contrast sensitivity (CS), a second visual function, evolved in a manner similar to BCVA: While values for GS010-treated eyes and sham-treated eyes remained below baseline, CS also recovered so that the gap to baseline diminished at Week 72 compared to Week 48.
RESCUE subjects will be evaluated again at 96 weeks, then data will be unmasked, allowing more detailed subject-level analyses to be conducted.
The third interventional study for GS010-REFLECT-is a randomized, double-masked, placebo-controlled Phase III trial evaluating the safety and efficacy of bilateral injections of GS010 in patients up to one year from onset of vision loss due to LHON. The first patient in REFLECT was treated in March 2018.
This week in optometry news: April 15, 2019
Optometry Times’ chief optometric editor named OD of the Year Optometry Times’ Chief Optometric Editor Benjamin P. Casella, OD, FAAO, has received this year’s OD of the Year award from the American Optometric Association (AOA). The award will be presented at Optometry’s Meeting 2019, June 19-23, in St. Louis, Missouri.

“We were delighted to hear that Dr. Casella was named Optometrist of the Year,” says Tom Ehardt, president of MultiMedia Healthcare, LLC. “Ben’s contributions have been instrumental in helping Optometry Times remain a timely and relevant source among optometrists. I know the editorial team joins me in congratulating him on this prestigious honor.”
A third-generation optometrist, Dr. Casella owns and operates Casella Eye Center in Augusta, GA, which was established in 1948 by his grandfather, Victor. After graduating from the University of Alabama at Birmingham School of Optometry and completing his residency at State University of New York College of Optometry, Dr. Casella joined the family practice in 2008, continuing the tradition of providing quality care to the local community alongside his father.
Dr. Casella is active within the professional optometric community, holding positions within the Georgia Optometric Association, serving on the AOA Evidence Based Optometry Committee, and having been named the Young OD of the South by SECO International in 2014.
“I am thrilled and humbled by this award,” Dr. Casella says. “I never expected such an honor, and I’m very grateful to my optometry families at the AOA, the GOA, and Optometry Times, as well as my own family for affording me the opportunities to play a small role in the advancement of our profession.”
The award is presented by the AOA, a professional association representing more than 44,000 optometrists, to one optometrist each year who goes above and beyond in providing outstanding service.

This week’s most popular story: Blog: 10 eyecare apps for more efficient patient careDr. Margie Recalde shares the top 10 mobile apps that can make an OD’s work life easier.Learn her top apps!
Alcon debuts as independent, publicly traded company
Alcon announced its debut as an independent, publicly traded company and the completion of its separation from Novartis on April 9, 2019. The company’s shares began trading that same day on the SIX Swiss Exchange and New York Stock Exchange (NYSE) under the symbol “ALC.”
According to the company, last year, Alcon had sales of $7.1 billion, including $4.0 billion in Surgical-up 7 percent from the prior year-and $3.1 billion in vision care-up 3 percent.
Under the terms of the separation, each Novartis shareholder or ADR (American Depositary Receipt) holder will receive one Alcon share for every five Novartis shares or ADRs they held as of the close of business on April 1, the record date for the distribution.

Top-read story: How to educate patients on risks of eyelash enhancementsDr. Bridgette Shen Lee says knowing lash options and talking with patients is key to better clinical results.See what she suggests!
AOA ODs, students rally on Capitol Hill
Hundreds of American Optometric Association (AOA) ODs, primary eye healthcare providers, and students from across the U.S. rallied in Washington D.C., to meet with lawmakers to discuss the expanding the role of eye doctors in health care and the importance of eye healthcare for all Americans.
“The tremendous growth of AOA on Capitol Hill-from fewer than 200 doctors attending a decade ago to more than 600 doctors and students this year-shows our commitment to advancing optometry’s pro-access, pro-patient priorities,” said AOA President Dr. Samuel D. Pierce.
The contingent emphasizes in-person eye health and vision care provided by ODs as well as the urgent need for policies putting patients first.
AOA's federal priority concerns addressed during meetings with lawmakers and staff in the House and Senate include:
• Abuses of health and vision plans
• Federal Trade Commission (FTC) contact lens mandate
• Simplified contact lens Rx verification
• Essential eye care for veterans
• Appropriate use of telehealth

Popular story: Sleep apnea: More than a snore and floppy eyelidsSome 18 million Americans suffer from obstructive sleep apnea syndrome (OSAS). Patients can be afflicted with OSAS and not be aware of the risk to their overall health. That is where clinical optometry comes in, especially for a patient who is falling asleep during a refraction.How ODs can help.
NECO professor releases OCT Visual Atlas app
OCTaVIA, a visual reference optical coherence tomography (OCT) app, is now available free on the Apple App Store.

The iOS OCT app was developed by Elena Z. Biffi, OD, MSc, FAAO, assistant professor at the New England College of Optometry (NECO) and an attending optometrist at South Boston Community Health Center, to assist in the diagnosis and management of retinal disorders in daily practice.
The OCTaVIA app provides eyecare professionals with normal OCT reference material and corresponding OCT with fundus photography images for multiple retinal disease conditions, as well as a brief disease-specific description of OCT images, high-yield key considerations, and useful links to aid in the differential diagnosis.
The intuitive search function provides access to dozens of retinal disease, allowing for quick access for students and doctors in busy clinical and teaching settings. Cases are presented in alphabetical order for easy offline access.
Within the app, users can find detailed normal images with reference to normal OCT layer-by-layer descriptions and nomenclature for retinal location and retinal OCT layer abnormalities.
The app was developed with financial support from the American Optometric Foundation and Johnson & Johnson Innovation in Education Grant. OCTaVIA is currently available for free on the Apple App Store for iPhone and iPad users.

Top-read story: New CA law affects all VSP providersA new law that will impact every contracted Vision Service Plan (VSP) provider-that most don’t even realize affects them-is a change in the California Health and Safety Code. It affects all VSP providers because the VSP contract (called the Network Provider Agreement or NDA) states that California laws apply to the VSP contract.Find out how.
Academy 2019, 3rd World Congress of Optometry education program released
The World Council of Optometry (WCO) and the American Academy of Optometry (AAO) are partnering to present an education program taking place at Academy 2019 Orlando and the third World Congress of Optometry, Oct. 23-27.
This year’s Plenary Session from 10 a.m. to noon on Oct. 23 is titled, “Today’s research, tomorrow’s practice: WHO World Report on Vision, Opportunities for Optometry to Make an Impact,” and will discuss the World Report on Vision on the distribution of eye disease and blindness across the globe and the disease burden these eye conditions pose on nations and regions.
Additionally, the human resource requirements of eyecare providers needed to address this public health crisis will be covered. Optometric representatives, specifically the session speakers, played an instrumental role in developing the report. They will discuss the findings and their implications for optometry internationally and for North America respectively. The keynote speaker from the World Health Organization (WHO) will give attendees an overview of the organization’s efforts to tackle the extensive disease and blindness burdens on society throughout the world, and where optometry fits into this effort.

Speakers include Kovin Naidoo, OD, PhD, FAAO, Sandra Block, OD, MPH, FAAO, and a speaker from WHO.
The Monroe J. Hirsch Research Symposium is titled, “Gene therapy for ocular and neurologic disorders.” Contemporary issues in gene therapy will be discussed, including Leber’s congenital amaurosis, animal models of glaucoma, and Leber’s hereditary optic neuropathy. Speakers will include Stephen Russell, MD, Abbott Clark, PhD, and Byron Lam, MD.
Ezell Fellows Present is a symposium where three investigators at different stages of their careers-and who were supported early on through the foundation’s Ezell Fellowships-will present their research.
This year marks the 10th anniversary of the symposium, titled, “Public Health/Epidemiology Potpourri.” Included in this session will be discussions on anterior segment infectious eye disease and U.S. national health datasets used to assess vision impairment.
Speakers will be Nicole Carnt, BOptom, PhD, FAAO, Charlotte Joslin, OD, PhD, FAAO, and Dean VanNasdale, OD, PhD, FAAO.
WCO’s President’s Forum is titled, “Optometry’s role in addressing the changing face of technology, public health and clinical care.”
This invitation-only event provides a platform wherein the highest level of decision makers and key partners of our profession come together to discuss the current development of optometry its intended impact in the broader health agenda across the world.
The Global Summit on Optometric Education, cosponsored by WCO, AAO, and the Association of Schools and Colleges of Optometry (ASCO), will provide educators from optometric educational programs around the globe the opportunity to share education philosophies/teaching methods and to discuss challenges facing institutions.
The meeting will take place at the Orange County Convention Center in Orlando. Registration and housing open on May 6.
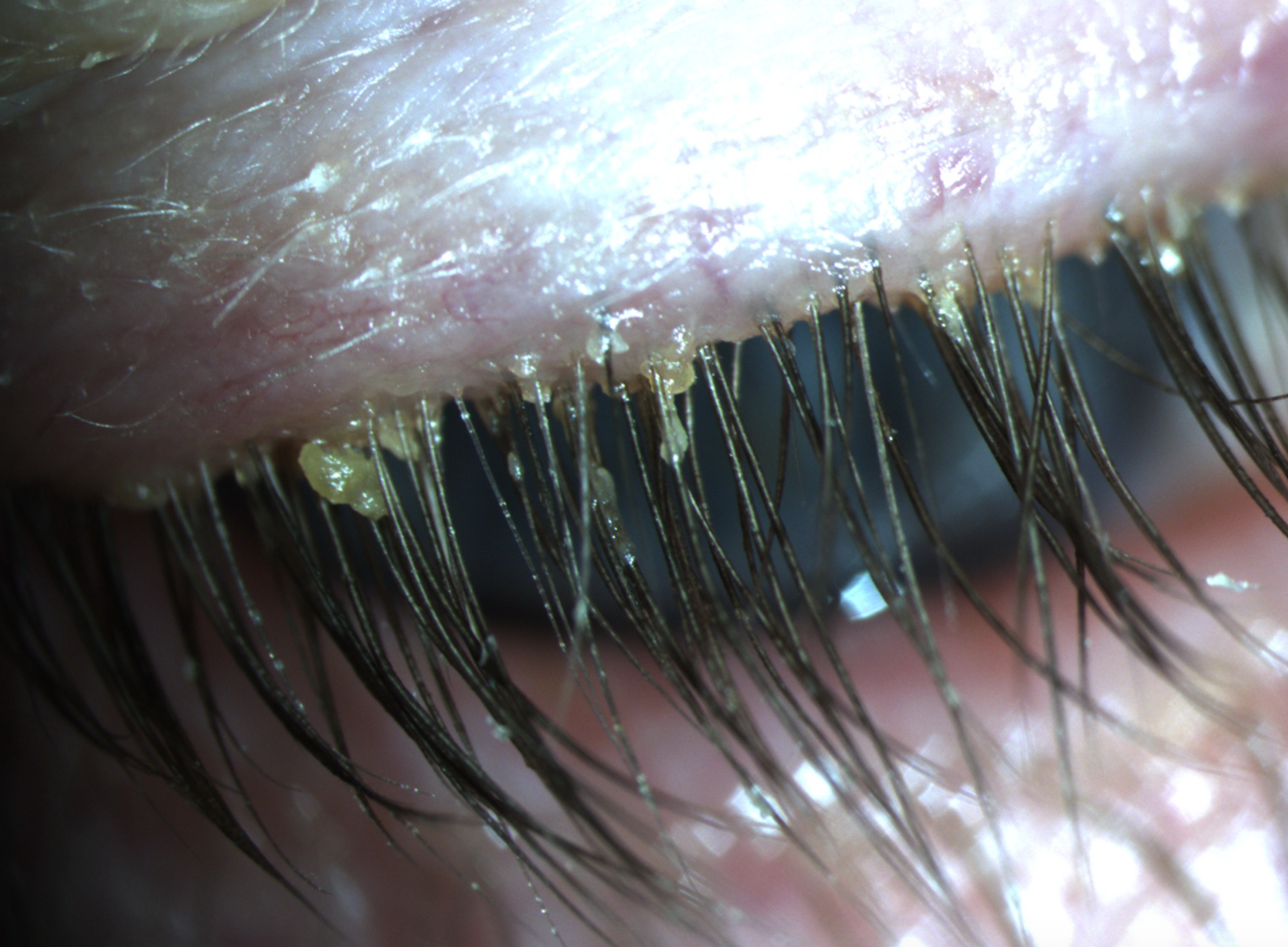
Top-read story: 4 steps to beating blepharitisNo matter the cause, the only way ODs can beat blepharitis is to determine the location as anterior, posterior, or mixed; differentiate among the causative factors, and treat according to the severity of the condition.Find out the steps.
NORA opens applications for grants, awards
The Neuro-Optometric Rehabilitation Association, International (NORA) announced it is offering up to 10 students and residents with an interest in neuro-rehabilitation an opportunity to apply for a student grant to attend the organization’s 2019 Clinical Skills Pre-Conference (Sept.19-20) and 28th annual General Conference (Sept. 21-22) in Scottsdale, AZ.
Each grant recipient will receive full conference registration and $400 in travel grant funds.

Vivid Vision is providing partial support for the program through a $1,000 donation.
To apply for the grant, students/residents must fill out an application, which includes a one to three-page essay/paper discussing their interest in neuro-rehabilitation and/or the values and principles of working as an interdisciplinary team with other professionals who provide rehabilitative services to individuals who have suffered an acquired brain injury. The application form should be mailed to NORAAwards@gmail.com. The deadline for submission is July 15. Winners will be notified by August 1.
In related news, NORA is accepting nominations until July 31 for the following awards, which acknowledge individuals and organizations for important contributions to the neuro-optometric rehabilitation area:
• Advancement of Neuro-Optometric Rehabilitation Award, presented to the individual, group, agency, or organization that has demonstrated treatments or clinical procedures to further the advancement of the art and science of neuro-optometric rehabilitation
• Advancement of Sciences Award, presented to the individual, group, agency, or organization that has provided a unique and valued contribution to the science of neuro-optometric rehabilitation
• Founding Father’s Award, given to those who demonstrate a life-long dedication to the exploration, elucidation, and enhancement of the concepts surrounding the nature of the human visual process
• William and Diana Ludlam Educators Award, presented to the individual educator or institution best exemplifying the commitment to serving the students and patients in the neuro-optometric rehabilitation effort
To submit a recommendation, visit https://noravisionrehab.org/about-nora/awards.

Popular story: The Vision Council hosts spring preview of eyewear releasesAs an extension of this year's Vision Expo East, The Vision Council offered an exclusive preview of this year's upcoming eyewear collections and optical industry's latest advancements in eyecare technology and health during a cocktail party at the Gramercy Park Hotel on Thursday, March 21.Take a look!
Asepticys files for 501k approval of TriFlect tech
Asepticys LLC, announced the successful completion of multi-site human clinical trials for the company’s contact lens care solution containing TriFlect technology, and the submission of its 510k application to the U.S. Food and Drug Administration (FDA).
The clinical trials involved over 320 patients at 12 sites across the United States. Asepticys' 510k application with the FDA marks a milestone toward commercial launch in the coming year of its first product using the TriFlect technology.
Nidek launches new technology
Nikon’s newest autorefractor, Auto Ref/Keratometer/Auto Refractometer (ARK-F/AR-F) features a fully-automatic measurement. By placing the chin on the chinrest, the eye detection camera automatically detects the position of the eyes, and measurement starts without pressing any button. Voice guidance allows for smooth measurement for any operator.
Because the screen can be continuously tilted and swivel, the ARK-F/AR-F can be placed anywhere in an examination area, including against a wall or in a corner of the room.
Freedom of operator mobility enables the support of a patient's eyelids during measurement.
Manual measurement using the large, durable 7.0-inch touch screen makes for easy alignment by long-pressing a position on the screen and an icon.
Clearly identifiable icons assure intuitive operation. Moreover, a newly designed hand-held control and tablet control software are available as options that increase operator freedom and productivity.
In related news, Nidek also released the RT-6100 Intelligent Refractor, designed to increase efficiency without compromising patient comfort and enhance refraction workflow.
The combination of a streamlined refractor head and user-friendly control console allows precise and quick examinations. Enhanced data functions create a seamless digital communications for various network structures.
The company has also released two new system table models: ST-6100 and ST-600.
This week in optometry news: April 8, 2019
IACLE announces global award winners in anniversary year
Contact lens educators from eight countries-Australia, China, India, Italy, Malawi, Malaysia, Mexico, and Taiwan-will receive awards from the International Association of Contact Lens Educators (IACLE) as it celebrates its 40th year.
IACLE has announced the winners of the 2019 IACLE Contact Lens Educator of the Year Awards to recognize and reward achievement in contact lens education, and the 2019 IACLE Travel Awards for members to attend major international meetings. This year’s awards will be presented at events around the world to mark IACLE’s 40th anniversary.
Sponsored by CooperVision-and cosponsored by the British Contact Lens Association (BCLA) and IACLE-three IACLE Contact Lens Educator of the Year Awards will be presented. Winners will travel to the BCLA Clinical Conference in Manchester, UK, at the end of May to receive their awards.

The 2019 IACLE Contact Lens Educators of the Year are:
IACLE Americas Contact Lens Educator of the Year
Rubén Velázquez
Universidad Nacional Autónoma de México, Mexico City
IACLE Asia Pacific Contact Lens Educator of the Year
Craig Woods
Deakin University, Victoria, Australia
IACLE Europe/Africa–Middle East Contact Lens Educator of the Year
Fabrizio Zeri
Università degli Studi di Milano-Bicocca, Milan, Italy
This year, five IACLE Travel Awards-funded by all IACLE’s industry sponsors (all awards), and cosponsored by BCLA (one award) and Association of Optometric Contact Lens Educators (AOCLE, one award)-will be presented.
The 2019 IACLE Travel Awards recipients and meetings they will attend include:
Joseph Afonne
Mzuzu University, Mzuzu, Malawi
BCLA Clinical Conference & Exhibition
Fakhruddin Barodawala
SEGi University, Petaling Jaya, Malaysia
Asia Pacific Optometric Congress
Maheswari Srinivasan
Shri Prakash Institute of Optometry, Chennai, India
AOCLE Annual Workshop, Pacific University, Oregon
Wan-Yun (Connie) Tsung
Central Taiwan University of Science and Technology, Taipei, Taiwan
Asia Pacific Optometric Congress
MA Yuying
Wuxi Occupational Institute of Arts and Technology, Wuxi, China
Asia Pacific Optometric Congress
This week’s most popular story: Educate, don’t sell to patientsIf you educate patients on why they need a certain product, they will better understand their own visual needs and how you, the doctor, can help meet those needs. They may also be more likely to support your practice. Read more.
Alcon adds Alcaine 0.5% 15 mL to ophthalmic solutions portfolio
Alcon announce the latest addition of Alcaine (proparacaine hydrochloride) 0.5% 15 mL to its procedural eye drops portfolio, which consists of mydriatic, cycloplegic, diagnostic, and anesthetic products.
Alcaine Solution 0.5% 15 mL, the only branded proparacaine available, is a topical local anesthetic indicated for corneal anesthesia of short duration, as well as short corneal and conjunctival procedures.
Alcon’s procedural eye drops portfolio includes:
• Tetracaine Hydrochloride Ophthalmic Solution 0.5% Steri-Units
• Isopto Atropine (atropine sulfate ophthalmic solution) 1%
• Cyclomydril (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution)
• Fluorescite (fluorescein injection) 10%
• Mydriacyl (tropicamide ophthalmic solution)
• Cyclogyl (cyclopentolate hydrochloride ophthalmic solution)
Top-read story: How air pollution affects the ocular surfacePatients come to ODs offices with dry eye complaints, and ODs point to factors such as age, dry air, increased digital device use, or systemic medications. But depending on where your practice is located, air pollution may be a cause of dry eye. Learn more.
DrChrono and Beam Health partner on new mobile app, EHR system for patients
DrChrono Inc. and Beam Health Group announced a partnership to integrate Beam Health’s mobile app with DrChrono’s EHR, giving physicians a means to upgrade their practices with the ability to offer patient appointments via smartphone.
Physician practices using DrChrono’s EHR platform can download Beam and immediately start seeing their patients via HD, HIPAA-compliant video consults. Beam also offers free marketing and serves as a tool to increase the frequency and volume of appointments with existing patients, as well as grow a practice’s reach to new patients.
Beam provides patients in rural and underserved areas access to primary-care doctors and specialists.
“Large integrated delivery networks (IDNs), health systems, and hospitals are purchasing, acquiring, and partnering with smaller individual offices across the country, making it increasingly difficult for private practitioners to thrive; the traditional telemedicine model has, in some ways, served to further this trend,” says Ranga Jayawardena, CCO and cofounder of Beam Health.
While most telemedicine companies are vertically integrated practices that hire physicians to provide remote consults, Beam offers providers a means to add telemedicine to their practices through the Beam Health mobile app, according to Jayawardena.
“With our integration into DrChrono, practices all over the country can now use the app within their EHR to immediately make telemedicine part of their practice,” he says.
Popular story: New CA law affects all VSP providersA new law that will impact every contracted Vision Service Plan (VSP) provider-that most don’t even realize affects them-is a change in the California Health and Safety Code. It affects all VSP providers because the VSP contract (called the Network Provider Agreement or NDA) states that California laws apply to the VSP contract. Find out how.
Alcon introduces new parameters for DACP toric contact lenses
Alcon has established a broader range of parameters for its Dailies AquaComfort Plus Toric contact lenses, giving it the most parameters of any other daily disposable contact lens for people with astigmatism, according to the company.
The expansion aims to provide eyecare professionals (ECPs) with access to a daily disposable toric contact lens offering coverage for 94 percent of astigmatic patients.
The new offering of 2,360 parameters includes -2.25 D cylinder, around-the-clock axes in core parameters, and expanded high-minus sphere powers.
Dailies AquaComfort Plus Toric contact lenses provide dual stability, including tear film and on-eye stability. This is achieved through the combination of blink-activated moisture technology that releases polyvinyl alcohol (PVA) with each blink for tear film stability. It also includes a dual thin-zone design, allowing both eyelids to apply equal pressure to keep the lens in the correct position.
April Fool: Online optometry school to launchBecause last Monday was April 1, we reminded everyone of our killer April Fool’s story from a few years ago. Read it!

DrChrono ranks as top mobile EHR for 7th year
DrChrono Inc has been ranked the number one mobile electronic health record (EHR) in 2019 by Black Book Research.
From Quarter 3 of last year through Quarter 1 of 2019, the Black Book Research electronic medical record (EMR), EHR, e-prescribing, practice management, and e-health client/user survey investigated 313 EMR vendors and validated 21,436 EMR users nationwide for rankings.
A total of 873 qualified mobile EHR physicians, practices, groups, and support staff participated in this year’s satisfaction survey. This year’s survey subset included 2,829 mobile EHR and practice management users.
Based on the 2019 Black Book survey results, DrChrono ranked as the number one mobile EHR for the seventh year in a row.
“Mobile EHRs are becoming a mainstay of medical practices from solo practitioners to enterprise medical facilities,” says Daniel Kivatinos, COO and co-founder of DrChrono. “Because we are a cloud-based mobile EHR platform with a medical API for healthcare app developers, we have the flexibility and built-in scalability physicians are looking for as they grow their practices.”
Top-read story: 4 steps to beating blepharitisNo matter the cause, the only way ODs can beat blepharitis is to determine the location as anterior, posterior, or mixed; differentiate among the causative factors, and treat according to the severity of the condition. Find out the steps.
Transitions Optical debuts customizable Eyeglass Guide
Transitions Optical unveils a revamped Eyeglass Guide, with a new tool customizable to each eyecare practice that allows patients to create their own pair of eyeglasses based on their vision needs, eyeglass style, and lifestyle preferences.
The many options available today can be overwhelming to patients. This guide will help them select frame styles and lenses that best fit their needs and preferences, according to the company.
The Eyeglass Builder tool includes eight questions that determine how often patients are bothered by light and glare, what activities they enjoy both indoors and outdoors, and their eyeglass persona.
The tool provides an image of the eyeglasses the patient designed as well as his personalized recommendation of lens options that he can print or email directly to the practice. Patients can also share the eyeglasses they designed on social media directly from the builder website.
Eyecare practices can sign up to personalize their own URL with their logo, practice name and contact information, as well as create links to automatically email results to the practice.
Eyecare practices interested in their own personalized Eyeglass Builder URL can sign up at www.eyeglassguide.com/create.
High-interest story: Sleep apnea: More than a snore and floppy eyelidsSome 18 million Americans suffer from obstructive sleep apnea syndrome (OSAS). Patients can be afflicted with OSAS and not be aware of the risk to their overall health. That is where clinical optometry comes in, especially for a patient who is falling asleep during a refraction. How ODs can help.
Bausch + Lomb launches Lotemax SM for postop inflammation, pain treatment
Bausch + Lomb has begun distributing its Lotexmax SM (loteprednol etabonate ophthalmic gel) 0.38% to U.S. pharmaceutical distributors.
The company received final approval by the U.S. Food and Drug Administration (FDA) on Feb. 22. Lotemax SM is a new gel drop formulation of loteprednol etabonate, which was designed with SubMicron (SM) technology for efficient penetration to key ocular tissues at a low preservative (BAK) level and a pH close to human tears, according to the company.
It is indicated for the treatment of postoperative inflammation and pain following ocular surgery.
Per Bausch + Lomb, Lotemax SM provides two times greater penetration to the aqueous humor as compared to Lotemax Gel (loteprednol etabonate ophthalmic gel) 0.5%.
Lotemax SM was formulated with:
• Moisturizing ingredients
• pH close to that of human tears
• Low BAK preservative percentage
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.